
May. 20, 2025

Identification of 2-(2-chlorophenyl)-2-nitrocyclohexanone in the Seized Drugs
WU Wenxian, XU Boyang, ZHANG Hongjian
Identification of 2-(2-chlorophenyl)-2-nitrocyclohexanone in the Seized Drugs
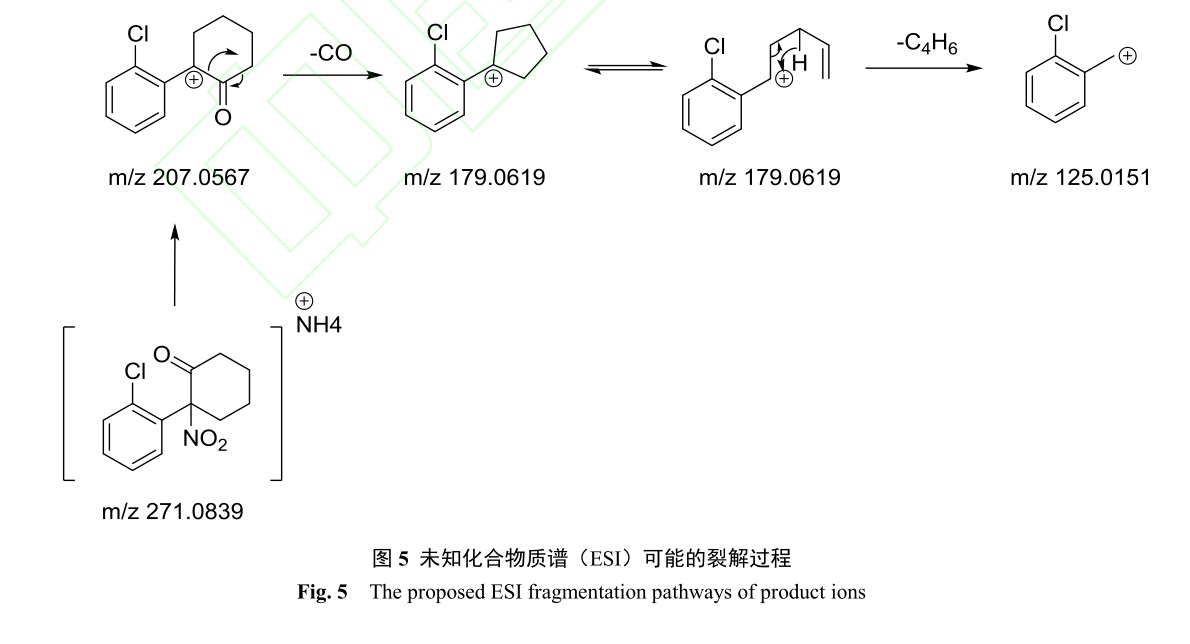
In October 2021, the Anti-drug Detachment of Wenzhou Public Security Bureau seized a package of yellow substances suspected of drugs in the adjacent sea area, and the case handling unit sent the yellow substances for inspection. To detail the composition of the sample and the structure of the main compound in the sample, it was analyzed by ultra-performance liquid chromatography tandem high resolution mass spectrometry (UHPLC-HRMS), nuclear magnetic resonance (NMR) and Fourier transform infrared spectrometer (FTIR). Initial testing indicated that the main compound of the sample was not matched in our in-house database, which prompted us to deeply analyze the unknown compound by different analytical techniques. The analysis of UHPLC-H RMS provided the precise mass quantity of the unknown compound with a mass accuracy of 2.5 ppm. The characteristic ions (m/z) were 125.0151, 179.0619 and 207.0567, close to those of ketamine, which indicated that the compound may be the analogue of ketamine. Proposed fragmentation mechanism is also present. Further analyses by 1H NMR, 13C NMR, 15N-NMR, distortionless enhancement by polarization transfer spectroscopy (DEPT 135°), proton two-dimensional correlation spectroscopy (1H-1H COSY), heteronuclear single-quantum correlation spectroscopy (HSQC), heteronuclear multiple bonding connectivity spectroscopy (HMBC) detailed the structure of the analogue. 15N-NMR confirmed the presence of nitro-group. DEPT pulse sequence utilized for the assignment of the different types of carbons showed that there were four methylene carbons and a quarternary carbon presented in the molecule of the unknown. Assignments were made via 1H NMR and 13C NMR, assisted by 1H-1H COSY, HSQC, and HMBC. IR has determined the type of such functional groups as carbonyl, nitryl, and chemical bonds of C and Cl by the related absorption characteristics. It was confirmed that the yellow powder was a new precursor 2-(2-chlorophenyl)-2-nitrocyclohexanone. According to the literature, it was found that it could be used to synthesize ketamine. It is the first time of this substance to be detected in suspected drugs in China. However, in recent years, the clinical interest in ketamine has increased due to its positive impact in treating depression and the rapid onset of its antidepressant effect. It led to an increase in publications of the procedure of the synthesis of ketamine, which may be used for illegal synthesis. 2-(2-chlorophenyl)-2-nitrocyclohexanone is an essential precursor of the new synthetic ketamine process for criminals to evade the attack, providing a reference for the control of precursor chemicals and the inspection of related cases in the future.
forensic toxicology / ketamine precursors / 2-(2-chlorophenyl)-2-nitrocyclohexanone / ultra-performance liquid chromatography tandem high resolution mass spectrometry (UHPLC-HRMS) / nuclear magnetic resonance (NMR) / Fourier transform infrared spectrometer (FTIR) {{custom_keyword}} /
Table 1 Exact masses of product ions of the sample and Ketamine表1 未知化合物与氯胺酮主要碎片离子峰对比(ESI+) |
| 名称 | 未知化合物 | 氯胺酮 | 相对相差/ppm | 元素组成 |
|---|---|---|---|---|
| 离子峰1 | 125.0151 | 125.0152 | 0.8 | C7H6Cl+ |
| 离子峰2 | 179.0619 | 179.0619 | 0 | C11H12Cl+ |
| 离子峰3 | 207.0567 | 207.0568 | 0.5 | C12H12ClO+ |
Table 2 NMR data of the sample in MeOD (δ in ppm)表2 未知化合物化学位移的归属 |
| 化合物结构 | 位置 | 碳谱 | 氢谱 |
|---|---|---|---|
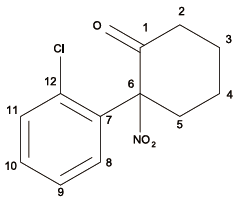 | 1 | 200.4 | / |
| 2 | 40.5 | 2.67-2.71,m | |
| 3 | 27.5 | 1.87-1.93,m、1.98-2.06,m | |
| 4 | 21.7 | 1.64-1.68,m、1.82-1.93,m | |
| 5 | 36.5 | 2.82-2.85,m,3.28-3.31,m | |
| 6 | 101.6 | / | |
| 7 | 131.7 | / | |
| 8 | 129.6 | 7.50,m | |
| 9 | 127.4 | 7.45,m | |
| 10 | 131.1 | 7.47,m | |
| 11 | 131.6 | 7.54,m | |
| 12 | 135.3 | / |
| [1] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [2] |
Ketamine, a molecule of many faces, has contributed immeasurably to numerous realms of clinical practice and scientific inquiry. From anesthesia and analgesia to depression and schizophrenia, it continues to shed light on the molecular underpinnings of pain, consciousness, and the pathophysiology of neuropsychiatric disorders. In particular, research on ketamine's mechanism of action is providing new hope in the search for therapies for treatment-resistant depression and affords insights into disorders of glutamatergic dysfunction. In this Review, we will cover aspects of ketamine's synthesis, manufacturing, metabolism, pharmacology, approved and off-label indications, and adverse effects. We will also discuss the captivating history of this molecule, its influence on neuropsychiatry, and its potential to advance the fields of chemical neuroscience and neuropharmacology.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [3] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [4] |
China National Narcotics Control Commission NNCC. Annual report on drug control in China[R]. 2018.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [5] |
公安部, 商务部, 海关总署, 等. 关于将邻氯苯基环戊酮增列为第一类易制毒化学品管理的公告[EB/OL]. (2012-09-17)[2022-02-26].
(The Ministry of Public Security of the People’s Republic of China, Ministry of Commerce of the People’s Republic of China, General Administration of Customs People’s Republic, et al. On the o-chlorphenyl cyclopentyl ketone added to the first class of precursor chemicals management notice[EB/OL]. (2012-09-17)[2022-02-26].
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [6] |
公安部, 商务部, 海关总署, 等. 关于将羟亚胺增列为第一类易制毒化学品管理的公告[EB/OL]. (2008-07-22) [2022-02-06]. http://www.gov.cn/zfjg/content_1052347.htm.
(The Ministry of Public Security of the People’s Republic of China, Ministry of Commerce of the People’s Republic of China, General Administration of Customs People’s Republic, et al. On the hydroxyimine added to the first class of precursor chemicals management notice[EB/OL]. (2008-07-22)[2022-02-06]. http://www.gov.cn/zfjg/content_1052347.htm.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [7] |
梁未未, 林贤文, 田源源, 等. 新型易制毒化学品α-乙酰基苯乙酸甲酯的结构确认和检验鉴定方法研究[J]. 刑事技术, 2021, 46(4):331-336.
目的 通过核磁共振波谱(NMR)确认一种新型易制毒原料的分子结构,并建立气相色谱-质谱联用(GC-MS)和衰减全反射红外光谱(FTIR ATR)的检验鉴定方法。方法 白色粉末样品用氘代氯仿溶解后进行核磁共振氢谱(<sup>1</sup>H-NMR)和碳谱(<sup>13</sup>C-NMR)测试,确认结构;样品用乙酸乙酯溶解后采用GC-MS检测;样品直接用FTIR ATR检测;样品在碱或酸环境中进行水解实验,并用GC-MS检测反应产物。结果 通过核磁共振波谱中的氢谱和碳谱确认了该物质的结构,分子式为C<sub>11</sub>H<sub>12</sub>O<sub>3</sub>,名称为α-乙酰基苯乙酸甲酯。GC-MS检测得样品中α-乙酰基苯乙酸甲酯保留时间为12.57min,主要的特征离子峰为m/z 43、90、118、150、192。FTIR ATR检测主要的特征峰为3068、3013、2960、2943、1738、1711。水解实验中,α-乙酰基苯乙酸甲酯在碱性环境中能100%转化为1-苯基-2-丙酮(P2P),酸性环境中95%转化为P2P。结论 本研究建立了新型易制毒化学品α-乙酰基苯乙酸甲酯的检验鉴定方法,首次成功验证了该化合物水解转化为P2P,为以后易制毒化学品的管制和相关案件的检验提供参考。
(
<strong>Objective</strong> To confirm with nuclear magnetic resonance (NMR) about the molecular structure of α-acetyl-methyl phenylacetate (a new precursor chemical for designer drug) that was found from a case, and to establish its identification through GC-MS detection plus an attenuated total reflection infrared spectrometer (FTIR ATR) approach for its qualitative analysis. <strong>Methods</strong> The white powder sample, seized from a case, was dissolved with deuterium chloroform, having its harboring chemical’s structure confirmed with the engendered <sup>1</sup>H-NMR and <sup>13</sup>C-NMR spectra. The sample was also dissolved into ethyl acetate to subject to GC-MS detection. Besides, FTIR ATR was adopted to have the sample tested. Furthermore, the sample was hydrolyzed under both alkali and acid environment, having the reaction products detected into GC/MS analysis. <strong>Results</strong> The structure of the chemical substance seized from the involving case was confirmed through NMR, showing its molecular formula: C<sub>11</sub>H<sub>12</sub>O<sub>3</sub> and systematic name: α-acetyl-methyl phenylacetate. With GC-MS detection, the α-acetyl-methyl phenylacetate was shown of its retention time 12.57 min, leaving the main characteristic fragment ions at <em>m</em>/<em>z</em> 43, 90, 118, 150, and 192. For FTIR ATR test, the α-acetyl-methyl phenylacetate revealed its main characteristic peaks at 3068, 3013, 2960, 2943, 1738 and 1711. Regrading to hydrolysis experiment, the α-acetyl-methyl phenylacetate can be 100% and 95% converted to P2P (1-Phenyl-2-Propanone) in alkaline and acidic environment, respectively. <strong>Conclusions</strong> The identification of α-acetyl-methyl phenylacetate (one new precursor chemical for designer drug) has been established here, having resulted in the first-time successful verification about hydrolysis of α-acetyl-methyl phenylacetate to P2P. The α-acetyl-methyl phenylacetate, presently an unregulated precursor for drug production, can therefore provide a reliable reference for its controlling and qualitative analysis with the discoveries here.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [8] |
赵阳, 胡羽鹏, 常颖, 等. MDMA前体PMK methyl glycidate的定性检验[J]. 刑事技术, 2022, 47(1):96-99.
目的 利用PMK methyl glycidate来合成胡椒基甲基酮(piperonyl methyl ketone,PMK),可进一步合成3,4-亚甲二氧基甲基苯丙胺(MDMA)。本文首次报道了中国大陆出现的PMK methyl glycidate,并应用气相色谱-质谱联用(GC-MS)和核磁共振(NMR)技术对化合物结构进行了分析与确证。 方法 样品分别用甲醇和DMSO-d<sub>6</sub>提取后,使用GC-MS和NMR进行检测。结果 通过GC-MS分析测得化合物的质谱特征碎片和保留时间信息,并对氢谱碳谱的信号峰进行归属,确定了化合物结构。 结论 该方法简便可靠,能用于PMK methyl glycidate的检验。
(
<strong>Objective</strong> To report the first-time emergence of PMK (piperonyl methyl ketone) methyl glycidate (a precursor substance of MDMA) in Chinese mainland through completion of establishing its gas chromatography-mass spectrometry (GC-MS) analytical method and confirming its structure with nuclear magnetic resonance (NMR) which to deliver the compound’s hydrogen and carbon spectrums. PMK methyl glycidate is able to synthesize MDMA (3,4- methylenedioxymethamphetamine, a controlled drug capable of seducing the eater into addiction) through transition of PMK. <strong>Methods</strong> The sample was extracted with methanol and DMSO-d6, successively subjected to detection of GC-MS and NMR. <strong>Results</strong> GC-MS rendered the characteristic mass-spectral fragments and retention time of the extracted compound with which NMR assigned the signal peaks of both hydrogen and carbon spectrums, having therewith determined the structure of the chemical substance. <strong>Conclusions</strong> At present, GC-MS technology, combined with mass spectrometric information database retrieval, is one of the most commonly used choices for analyzing drugs and their precursors in forensic laboratories. However, due to the difficulty in obtaining available reference substances against the combination of some drugs and their precursors, the absolute accuracy of retrieval results cannot be guaranteed. NMR approach is independently eligible to confirm the structures of compounds of the reference substances so as to ensure the accuracy of the identification results. In this study, PMK methyl glycidate was identified with GC-MS and NMR, having its <sup>1</sup>H-NMR and <sup>13</sup>C-NMR spectra analyzed, thus providing a reference for identification of other similar compounds. The method is simple, reliable, and suitable for qualitative analysis of PMK methyl glycidate.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [9] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [10] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [11] |
刘翠梅, 韩煜, 闵顺耕. 甲基苯丙胺、氯胺酮、海洛因、可卡因红外光谱快速定性分析方法研究[J]. 光谱学与光谱分析, 2019, 39(7): 2136-2141.
(
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [12] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [13] |
张丽娟, 李宝璋. 邻-氯苯基环戊基酮合成法的改进[J]. 中国药物化学杂志, 1995(1): 47-48.
(
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
Supplementary files
补充材料 cs20220402001(吴文贤)缴获物中2-(2-氯苯基)-2-硝基环已酮的检验鉴定.pdf (730990 KB)
 Fig.1 The structure of 2-(2-Chlorophenyl)-2-nitrocyclohexanone
Fig.1 The structure of 2-(2-Chlorophenyl)-2-nitrocyclohexanone Fig.2 Mass spectra of the sample by UHPLC-HRMS in the MS1 mode
Fig.2 Mass spectra of the sample by UHPLC-HRMS in the MS1 mode Table 1 Exact masses of product ions of the sample and Ketamine
Table 1 Exact masses of product ions of the sample and Ketamine Fig.3 Mass spectra of the sample by UHPLC-HRMS in the MS2 mode (a: 276.0391; b: 271.0839)
Fig.3 Mass spectra of the sample by UHPLC-HRMS in the MS2 mode (a: 276.0391; b: 271.0839) Fig.4 FTIR ATR spectrums of the sample
Fig.4 FTIR ATR spectrums of the sample Table 2 NMR data of the sample in MeOD (δ in ppm)
Table 2 NMR data of the sample in MeOD (δ in ppm) Fig.5 The proposed ESI fragmentation pathways of product ions
Fig.5 The proposed ESI fragmentation pathways of product ions Fig.6 Synthesis of Ketamine by 2-(2-chlorophenyl)-2-nitrocyclohexanone
Fig.6 Synthesis of Ketamine by 2-(2-chlorophenyl)-2-nitrocyclohexanone/
| 〈 |
|
〉 |