第一作者简介:舒翠霞,女,安徽宣城人,硕士,警务技术四级主管,研究方向为毒物分析。E-mail: shucuixia7@163.com
具有镇静催眠作用的氟硝西泮曾在世界范围内被滥用,常被用于自杀、谋杀、迷奸、迷抢等案(事)件。近年其又成为俱乐部滥用药物之一。该药物在体内主要经肝脏代谢为7-氨基氟硝西泮和N-去甲基氟硝西泮,且7-氨基氟硝西泮的血药浓度常常大于血液中的母体药物浓度, 大约90%的代谢产物经尿液排出,10%经粪便排出。目前报道的相关分析方法主要是对于血液、尿液、毛发、酒水饮品等检材经LLE、SPE、LPME等净化萃取后,采用毛细管电泳法、色谱法、质谱法及各种技术的联用,检测母体药物及相关代谢产物。本文对氟硝西泮的滥用、体内代谢以及样品的提取净化、仪器分析等进行总结,为相关案(事)件的办理提供参考。
Flunitrazepam, one kind of benzodiazepine-categorized drugs, is effectual of pharmacological sedation and hypnosis, yet being worldwide abused into suicide, murder, drugging to sexually assault and/or loot. In recent years, it has also become one of the popular nightclub drugs in conjunction of other drugs and particular the alcohol, resulting in prominent toxic effects of wide social concern. Flunitrazepam is almost completely metabolized through liver, having brought forth two major metabolites: 7-aminoflunitrazepam and N-desmethylflunitrazepam. The 7-aminoflunitrazepam can greatly exceed the concentration of its unchanged parent drug in blood or plasma samples, leaving approximate 90% of its metabolites to be excreted through urine and the rest 10% into feces. The flunitrazepam and its metabolites can be detected from specimens of blood, plasma, urine, hair and others with various methods including capillary electrophoresis, mass spectrometry, chromatography and the combination of relevant techniques. GC-MS is a sensitive and specific choice for detection of flunitrazepam whose metabolites are yet required to derivatize prior to analysis. HPLC allows the simultaneous separation of flunitrazepam and its metabolites without prior derivatization, being highly recognized of its coupling with MS detection to result in better sensitivity and specificity than with UV detection. This paper summarizes flunitrazepam of its poisoning from abusing, metabolism in vivo, and the detection technologies about itself and metabolites, aiming to provide references for peers and investigation of relevant cases.
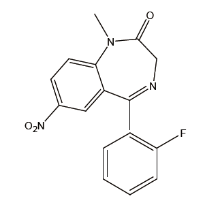
氟硝西泮(结构式如图1)又称氟硝基安定, 淡黄色结晶性固体, 微溶于水, 易溶于乙醇, 属于苯二氮卓类药物, 该药物具有催眠、遗忘、镇静、抗焦虑、肌肉松弛和抗惊厥作用, 其中催眠和遗忘作用更显著, 临床主要用于催眠和术前镇静。氟硝西泮于1980年在瑞典上市, 目前已有2 000多个品种, 是苯二氮卓类中使用最广泛的处方药, 这些药物的药理性质相似, 主要区别在于作用强度、作用速度以及作用持续时间不同。
随着越来越多的人走进各种俱乐部, 用于娱乐的新型毒品也不断在俱乐部蔓延, 例如当前市面流行的娱乐性药物“ 蓝精灵” 。“ 蓝精灵” 中主要药物成分是氟硝西泮, 服用后会使人神经兴奋, 产生幻觉, 在高剂量的情况下, 这种药物会导致肌肉缺乏控制和顺行性遗忘, 其他副作用包括低血压、头晕、神志不清和攻击行为等。由于氟硝西泮中毒后具有暴力行为且常常成为杀人[1]、迷奸[2, 3]等案件的犯罪工具, 美国早在1992年已将其列为违禁药物, 在我国被列为第二类精神药品。
在许多国家, 氟硝西泮广泛用于失眠患者, 但也在酗酒和吸毒人群中流行。在瑞典氟硝西泮涉及了多起致命的中毒案件[1], 据报道, 1992~1998年瑞典某法医化学系检测的案件中, 有641起死亡案与氟硝西泮中毒有关, 其中死亡原因单纯由氟硝西泮中毒引起的案件有130起, 氟硝西泮与其他药物联合使用中毒, 或者作为伴随条件的案件有511起。服用氟硝西泮后人会变得更健谈、更放松、更舒适, 或者自我感觉强大, 自尊心变强, 恐惧感减少, 会对琐碎的事情表现出愤怒; 中毒时容易变得好斗, 可能犯下抢劫和杀人的罪行, 并且具有顺行性遗忘。氟硝西泮中毒的人具有暴力和过度自信等表现, 被称为“ 兰博综合征” [4]。氟硝西泮中毒时引发的暴力案件易与精神病犯罪产生混淆, 因此, 公安机关在办理一些意想不到的攻击、杀人后顺行性失忆症的案例时应及时评估是否滥用氟硝西泮, 正确评估精神疾病与氟硝西泮中毒。
氟硝西泮常常成为犯罪嫌疑人实施迷奸的工具, 曾一度被称为“ 约会强奸药” 和“ 迷奸药” 。根据国内外流行病学调查, 涉及性侵药物比例呈上升趋势[5, 6, 7], 2018年我国台湾省高雄医科大学对台湾中南部近几年126份性侵受害者尿样进行检测, 29例(23%)检材检出一种或多种药物阳性, 最常见的药物是氟硝西泮(11.1%)。氟硝西泮通常与乙醇合并滥用, 滥用后受害者在药物作用下无能力反抗而被强奸, 并产生顺行性遗忘, 对所发生的事情失去记忆。
娱乐消遣使用氟硝西泮中等剂量(1.25 mg)可产生愉快与不愉快模糊不清的感觉, 其在俱乐部娱乐消遣使用产生的毒性反应已引起广泛关注。使用氟硝西泮发生高危险因素主要是与其他药物合用, 如氟硝西泮可显著增加美沙酮和丁丙诺啡的毒性, Rickert 等[8]调查900名服用氟硝西泮的女性患者, 合并使用乙醇的致死率比单用氟硝西泮高 1/3, 合并使用其他药物的致死率比单用氟硝西泮高1倍。因此, 快速、准确测定生物样品中氟硝西泮成分在侦查办案过程中对确定案(事)件性质、分析作案手段、提供诉讼证据等具有不可替代的作用。目前该类毒品主要通过网购、海淘、代购等途径获得, 犯罪活动更趋隐蔽、打击难度更大。
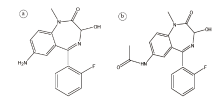
氟硝西泮药代动力学特性主要表现为起效迅速和作用时间中等, 它与苯二氮卓类受体有很高的亲核性因而可快速吸收。氟硝西泮口服后80%~90%经胃肠道吸收, 几乎完全由肝脏代谢, 氟硝西泮在体内代谢广泛, 其反应途径主要包括苯环上的N-还原反应, 其次是羟基化、去甲基化、乙酰化、羧基化、葡萄糖醛酸化以及上述反应的结合, 这些反应可产生多种代谢产物, 其中主要代谢产物是7-氨基氟硝西泮(结构式如图2a)和N-去甲基氟硝西泮, 7-氨基氟硝西泮由于体外还原作用, 其浓度大大超过血液或血浆中的母体药物浓度[9]。在氟硝西泮相关死亡案件中, 常常发现较高浓度的7-氨基氟硝西泮, 而母体药通常检测不到[10, 11]。有研究指出[4], 口服2 mg氟硝西泮后, 4 h可在血液中检测到原药, 12 h后可检测到其主要代谢产物, 并且在此后的5~21 d均可在尿液中检测到氟硝西泮及7-氨基氟硝西泮。Hasegawa等[12]对氟硝西泮中毒死亡人的心血、胆汁、肝脏、脾脏、胰腺、肾脏等组织进行检测, 发现大多数标本中7-氨基氟硝西泮浓度普遍高于母药氟硝西泮的浓度, 7-氨基氟硝西泮浓度最高是在胆汁, 其次是肾脏、胰腺、心血、脾脏和肝脏, 而氟硝西泮在胰腺中浓度最高, 其次是脾脏、胆汁、心血和大脑, 脂肪组织中氟硝西泮与7-氨基氟硝西泮浓度含量均较低。近期有报道Qin 等[13]在尿液中检测出25种氟硝西泮的代谢产物, 并且发现由7-氨基氟硝西泮乙酰化和羟基化得到物质M12(化学结构如图2b)浓度明显高于其余代谢产物, 指出该物质可作为一种潜在新的生物标志物来监测氟硝西泮滥用情况。口服氟硝西泮后大约90%经尿液排出, 10%经粪便排出。因此在处理氟硝西泮相关案件中应尽可能早地收集血液、尿液等检材样品, 对氟硝西泮及其代谢物进行定性、定量检测。
近年有关氟硝西泮的相关检测报道主要涉及血浆[14]、全血[15, 16]和尿液[17]中目标物的分析报道。由于氟硝西泮具有顺行性遗忘作用, 半衰期较短, 加之迷奸相关案件举报犯罪延迟等, 在部分案件中血液、尿液等生物样品中目标物已代谢消除, 经典的生物样品血液、尿液失去检测意义, 就此有实验室开发了基于毛发中氟硝西泮及其代谢物7-氨基氟硝西泮的检测分析方法[18, 19]。也有研究者考虑到涉及案(事)件的饮料、酒水等物证中氟硝西泮的检测也可支持其他证据, 因此有人开展了饮料、酒水等饮品中氟硝西泮的检测及稳定性考察[20, 21], 结果显示氟硝西泮有降解, 因此在办理相关案件时应尽可能早收集、分析与案件有关的饮品物证, 同时应谨慎解释长期储存样本的定量结果。
涉及氟硝西泮检测分析的前处理研究中, 大多数采取液液萃取(LLE)、固相萃取(SPE)、液相微萃取(LPME)和沉淀蛋白等。液液萃取法有碱性条件下用苯-异戊醇(98.5:1.5)提取[22]。固相萃取中有采用Oasis MCX柱萃取血浆中氟硝西泮及其代谢物, 该方法用甲醇和水进行柱活化、平衡; 上样后用0.1 N的盐酸和甲醇淋洗, 二氯甲烷/异丙醇/氨(78:20:2)洗脱, 有关氟硝西泮及其代谢物低、中、高三浓度的萃取回收率均大于90%[3]。也有报道采用C18柱提取血浆、尿液中氟硝西泮及其代谢物, 用甲醇、水进行柱活化、平衡; 上样后, 水淋洗, 三氯甲烷洗脱。该方法在血浆、尿液中萃取回收率均大于83%[23]。De Boeck等[24]在碱性条件下对全血中包括氟硝西泮在内的苯二氮卓类药物进行分散液液微萃取, BMIMPF6离子液体作为萃取溶剂, 低、高浓度的氟硝西泮回收率分别为117.7%和124%。固相萃取技术是公认的标准, 但其缺点是劳动密集、耗时长; 分散液液微萃取快速、经济。Hasegawa等[12]采用乙腈沉淀蛋白, 经QuEChERS方法净化, 提取胆汁、脂肪组织、肾组织等生物样品中氟硝西泮及其代谢物, 回收率在64.5%~104%之间。上述萃取模式通常是离线模式下进, Masaya等[25]采用Monolithic SPE SpinTip在线模式方法分别萃取20 µ L血浆和100 µ L尿液中氟硝西泮及其代谢物, 血浆和尿液中的回收率分别为93.5%~118%、97.7%~109%, 该方法与LLE及传统固相萃取方法相比, 缩短了萃取时间, 减少溶剂消耗及减低柱萃取堵柱概率。
现有文献报道中, 有气相色谱-质谱联用(GC-MS)法[26, 27]、毛细管电泳法[28]、高效薄层色谱(HPTLC)法[29]、高效液相色谱(HPLC)法[23]、高效液相色谱-质谱(HPLC-MS)法[30, 31]或HPLC-MS/MS[12, 32]及电化学分析法[33]测定不同基质中氟硝西泮及其代谢物含量。GC-MS对氟硝西泮的检测和定量具有很高的敏感性和特异性, 主要缺点是代谢物分析前需要衍生化, 耗费时间[34, 35]。与GC-MS相比, HPLC不需要衍生化即可同时分析氟硝西泮及其代谢产物, 但HPLC结合紫外检测器特异性差, HPLC-MS可增加其灵敏度, HPLC-MS/MS可进一步提高特异性, 增强信噪比。
Elian等[35]利用GC-MS同时分析血液和血斑中氟硝西泮及其代谢物7-氨基氟硝西泮、N-去甲基氟硝西泮, 该方法先用五氟丙酸酐(PFPA)衍生化7-氨基氟硝西泮, 再用含有1%的叔丁基二甲基氯硅烷(TBDMSCI)的三氟乙酸铵(MTBSTFA)衍生化N-去甲基氟硝西泮, 并且二次衍生7-氨基氟硝西泮。该方法可改善色谱峰并且提高灵敏度, 1 mL血液的检测限为0.1 mg/dL。吴玉红[29]采用HPTLC方法定性和半定量分析尿液中7-氨基氟硝西泮, 检测限为5 μ g/L, 测量限为15 μ g/L。Masaya等[25]采用超高效液相色谱-四极杆飞行时间质谱(UPLC-Q-TOF-MS)法分析血浆和尿液中氟硝西泮和7-氨基氟硝西泮, 采用电喷雾电离, 正离子模式进行检测, 检出限在0.2~0.5 ng/mL, 总的色谱分析时间为5 min。Qin等[13]采用液相色谱-静电场轨道阱高分辨质谱仪(LC-QE-HF-MS)检测到25种氟硝西泮代谢物, 该方法采用Hypersil gold C18柱, 流动相为水(含0.1% 甲酸)和乙腈(含0.1% 甲酸), 梯度洗脱, 流速为0.3 mL/min, 色谱分析时长为8.5 min; 质谱采用电喷雾电离源, 全扫描和数据依赖性二级质谱扫描模式。Kollroser等[14]利用高效液相色谱-大气压化学电离-串联质谱(HPLC-APCI-MS-MS)方法同时分析血浆中氟硝西泮及其主要代谢物7-氨基氟硝西泮、N-去甲基氟硝西泮, 该方法采用C18柱, 乙腈和0.1%的甲酸作为流动相, 梯度洗脱, 流速为0.6 mL/min, 氟硝西泮及其主要代谢物7-氨基氟硝西泮、N-去甲基氟硝西泮检出限分别为0.25、0.5和2.0 µ g/L。近期报道中[33]有采用氧化铜复合多壁碳纳米管修饰电极检测血清、尿液和药物中氟硝西泮的方法, 并对电解液pH、扫描速率等实验条件进行优化测试, 在最佳条件下, 氟硝西泮在0.05~346.6 µ mol/L范围内呈线性关系, 最低检出限为14.3 nmol/L。
具有镇静催眠作用的氟硝西泮滥用问题在国外研究报道较多, 近年含有氟硝西泮的“ 蓝精灵” 新型毒品问题在我国开始凸显, 该类毒品借助互联网技术和现代物流技术, 犯罪活动趋于隐蔽, 打击难打大, 因此需要加强该类新型毒品的宣传和打击。同时, 对氟硝西泮的检测分析需要进一步研究, 需要有效结合前处理技术和检测技术, 建立净化效果好, 污染少, 灵敏性、特异性、分离效率高, 且适合基层办案单位需求的简便分析方法。例如免疫分析方法特异性强、灵敏度高, 适合大量毒品的筛查, 并可降低假阴性概率, 有较好的应用前景。除此之外, 国内外有关氟硝西泮的动物实验研究报道很少, 有关代谢物的文献数据非常有限, 因此急需开展相关动物实验研究, 填补这一领域的空缺。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|