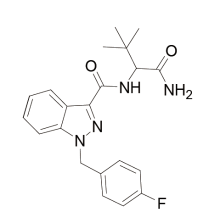
目的 新精神活性物质在世界范围内蔓延迅速,但相关标准物质的短缺制约着其分析方法的研究和案件检验。本文以我国首次出现的N-(1-氨甲酰基-2,2-二甲基丙基)-1-(4-氟苄基)吲唑-3-甲酰胺(ADB-FUBINACA)制毒案件为例,介绍在无法及时获得商业化标准物质的情况下,不得不通过自主合成制备标准物质解决案件检验难题,建立该新精神活性物质检验的方法。方法 建立气相色谱-质谱检验方法,分析条件:色谱柱为Aglient DB-5MS石英毛细管柱(30.00m×0.25mm×0.25μm);初始柱温60℃,按15℃/min升至300℃,保持15min;载气为氦,流速1.0mL/min,分流进样,进样量1.0μL,分流比20:1;进样口温度280℃;电子轰击(EI)离子源,电子能量70eV,离子源温度230℃,四级杆温度150℃,传输线温度280℃,质量扫描范围 m/ z 40 ~ 500amu,全扫描模式(SCAN)采集总离子流图,溶剂延迟3.0min。案件缴获的送检未知样品经甲醇提取,超声、离心后,取上清液以GC-MS分析;将所得主要质谱特征碎片峰( m/ z)通过NIST质谱库、SWGDRUG质谱数据库以及相关文献进行检索,初步确定待测目标物。采用有机合成技术制备ADB-FUBINACA标准物质,合成路线为:吲唑-3-甲酸甲酯与4-氟苄溴发生取代反应,生成1-(4-氟苄基)-1H-吲唑-3-甲酸甲酯;取代产物在碱性条件下经水解反应得到有机酸1-(4-氟苄基)-1H-吲唑-3-甲酸;在催化剂作用下,有机酸与L-叔亮酰胺发生酰化反应,制得化合物ADB-FUBINACA。经气相色谱-质谱(GC-MS)、液相色谱-离子阱-飞行时间质谱(LCMS-IT-TOF)、核磁共振(NMR)等分析,合成化合物的结构得以确证;同时采用超高效液相色谱-二极管阵列检测器(HPLC-PDA)进行分析,对其归一化纯度进行测定。将案件未知样品和合成标准物质分别用甲醇提取,超声、离心后,再行上清液GC-MS分析。结果 经GC-MS分析,案件未知样品(RT=19.818min)的质谱特征碎片峰( m/ z)信息为109.0 (基峰)、253.1、338.1、309.1和145.0,经与合成标准物质的保留时间及质谱图检测比对,证实为N-(1-氨甲酰基-2,2-二甲基丙基)-1-(4-氟苄基)吲唑-3-甲酰胺;通过查阅相关资料,对上述质谱特征碎片峰的产生机制进行了推断,并对1H-NMR、13C-NMR和DEPT-135等一维核磁谱图的信号进行了归属分析。结论 本文报道的新精神活性物质ADB-FUBINACA其GC-MS分析方法,可用于实际案件检验鉴定;合成化合物的结构表征方法也可用于固体ADB-FUBINACA的定性分析。基于有机合成技术的新精神活性物质制备与案件检验方法,可缓解有关标准物质短缺制约该类案件检验鉴定的现状。
Author: ZHAO Yanbiao, male, Baoding of Hebei, master of science, mainly focusing on the detection of illicit drugs. E-mail: zhaoyanbiao2011@163.com
New psychoactive substances (NPSs), mainly from synthetic cannabinoids, are of detrimental impact on public security worldwide. The dramatically ever-increasing NPSs cause the available reference standards unable to meet the demand with forensic identification, posing enormous challenges for forensic scientists. This article is about a successful countermeasure against the occurrence of such one challenge. Tiny amount of viscous material seized from an illicit clandestine manufacturer was analyzed through gas chromatography mass spectrometry (GC-MS), therewith having been suspected of containing synthetic cannabinoid ADB-FUBINACA, one NPS. However, the identification cannot be accomplished due to no timely available reference standard. Hence, a reference standard was synthesized of the ADB-FUBINACA in our laboratory. The synthesized chemical was essentially confirmed as the ADB-FUBINACA through GC-MS, liquid chromatograph mass spectrometer-ion trap-time of flight (LCMS-IT-TOF), nuclear magnetic resonance spectroscopy (NMR) and high performance liquid chromatography-photo-diode array (HPLC-PDA), with its purity being detected of 98.7%. Under the identical experimental conditions, both the seized material and the synthesized ADB-FUBINACA were compared through GC-MS analysis. The seized material was finally identified of harboring N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide, the ADB-FUBINACA, indeed.
According to the World Drug Report 2019 [1], synthetic cannabinoids are the most dramatically growing class of new psychoactive substances (NPSs), having their trafficking and abuse become a worldwide issue. Such substances act on human cannabinoid receptors type 1 (CB1) and 2 (CB2), resulting in relevant psychoactive effects [2]. Typically, synthetic cannabinoids are described as “ legal high” products, often sold through internet routes. Of the seized materials related, the most prevalent forms are ready-to-smoke mixtures of plant or chemical materials containing synthetic cannabinoid additives (such as herbal incenses, tobacco, e-cigarette liquid among others) [3, 4], with the second ubiquitous form being the powder of packaging pure synthetic cannabinoid. Yet, there are increasing presence of solid/liquid-form semi-products of synthetic cannabinoids that are ferreted out in clandestine dens.
Aiming to fight against the crimes involving with synthetic cannabinoids, forensic scientists have continuously done extensive efforts to identify the emerging NPS. United Nations Office on Drugs and Crime (UNODC) has summarized and recommended a lot of methods and approaches for identification and analysis of synthetic cannabinoids [5]. The authoritative forensic organizations, e.g., Scientific Working Group for Analysis of Seized Drugs (SWGDRUG) [6] and Southern Association of Forensic Scientists (SAFS) [7], are contributing to establish NPS’ spectrum databases. Excellent chemical companies have developed and supplied great many relevant reference standards. However, the technical requirements of identification, such as methodologies, analytical equipment, are usually not available to any forensic laboratory, with the reference standards being among the most inconvenience.
Confronted against the ever-increasing emergence of synthetic cannabinoids, forensic scientists are very likely to encounter such dilemmas where the suspected synthetic cannabinoid is difficult to procure its reference standard, leading the identification unable to go forward. Of course, the suspected synthetic cannabinoid, if pure and/or amount enough (capable of extracting from the sufficient material seized), can be analyzed with appropriate technical means, such as GC-MS, HRMS and NMR, to have its structure elucidated and verified [8, 9]. Nevertheless, there are usual cases of only tiny amount of seized materials in form of herbal mixtures or chemical components. Such the intractable seized materials cannot produce substance pure enough for structural elucidation. The involving analysis and identification could thus be hampered from the lack of reference standard.
One seized viscous material was sent to our laboratory for investigation of an illicit drug manufacturing case. With the analysis of gas chromatography-mass spectrometry (GC-MS), the synthetic cannabinoid ADB-FUBINACA (Fig.1) was suspected of presence. Yet, no timely available reference standard had left the identification in stopping. Hence, a reference standard of ADB-FUBINACA was to synthesize to enable identifying the seized material. An analytical combination (GC-MS, LCMS-IT-TOF, NMR and HPLC-PDA) was adopted for confirming the structure of the synthesized reference standard. Finally, the unequivocal identification was achieved of the suspected synthetic cannabinoid as ADB-FUBINACA in the seized yellow viscous material. As far as our knowledge, it should be the first time that a reference standard was synthesized in a forensic laboratory just for the only purpose of identification on an emerging synthetic cannabinoid.
In September 2018, a yellow viscous seized material, weighing less than 100 mg, was asked for us to have it analyzed. The material was seized in a clandestine illicit drug manufacturer, being guessed of a failure semi-product of synthetic cannabinoids.
All solvents used were of HPLC grade. Methanol and acetonitrile are products from Fisher Chemical (Fisher Geel, Belgium). Water was purified from a Milli-Q system of Merck Millipore (Molsheim, France). Formic acid, of mass spectrometry grade, was purchased from Sigma-Aldrich (Shanghai, China). Deuterated chloroform (0.03%, v/v tetramethylsilane) for NMR was obtained in ampoules (750 mL) from Sigma-Aldrich (Saint Aubain, France). The regents and solvents for synthesis and purification were of analytical grade. Obtainment from J& K Scientific (Beijing, China) and Aladdin (Shanghai, China) was indazole-3-carboxylic acid methyl ester, 4-florobenzyl bromide and L-tert-leucin amide hydrochloride.
2.3.1 Analysis of the seized material through gas chromatography-mass spectrometry (GC-MS)
For GC-MS analysis, 10 mg of the seized viscous material was added with 10 mL of methanol, afterwards subjected to ultrasonic extraction and centrifuged at 5 000 r/min for 5 min with a Sigma 3K15 centrifuge (Sigma, Osterode am Harz, Germany). The supernatant was analyzed with an Agilent 7890 B gas chromatograph combined of an Agilent 7693 injector and an Agilent 5977B inert mass selective detector (Agilent, California, USA). The chromatographic separation was conducted on a DB-5MS column (30 m × 0.25 mm, 0.25 μ m film thickness, Agilent, California, USA). Helium was the selected carrier gas at a constant flow of 1.0 mL/min. Oven temperature was programmed from 60 ° C to 300 ° C at a stepping rate of 15 ° C/min and held for 15 min, with the total running time of 31 min. The temperatures of the injection port and the detector were set at 280 ° C and 230 ° C, respectively. The transfer line temperature was set at 280 ° C. The injection volume was 1 μ L under the splitting injection mode at a split ratio of 20:1. The scan range for the mass spectrometer was m/z 40~500amu. The obtained mass spectra of the suspected substance were compared to EI-MS spectra libraries (NIST) and an on-line library (SWGDRUG monographs).
2.3.2 Synthesis of ADB-FUBINACA
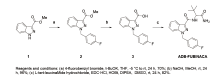
The ADB-FUBINACA was synthesized according to the published elsewhere [9, 10, 11] plus modifications, being schematically shown in Fig.2. The synthetic process was as follows. Indazole-3-carboxylic acid methyl ester 1 was deprotonated with potassium tert-butoxide and alkylated with 4-florobenzyl bromide to afford the methyl 1-(4-fluorobenzyl)-1H-indazole-3-carboxylate 2 (a little yellow solid). In the presence of sodium hydroxide, base-catalyzed hydrolysis of the methyl ester 2 gave the 1-(4-fluorobenzyl)-1H-indazole-3-carboxylic acid 3 (a white solid). Finally, amide bond was formed through free acid 3 subjecting to HOBt/EDC coupling with L-tert-leucinamide to give birth of the ADB-FUBINACA (a white powder) after the purification with flash chromatography. The information of the compounds 2 and 3 has already had descriptions somewhere [11]. Additionally, the stability was tested under different conditions, showing that the synthesized ADB-FUBINACA powder was stable within a year under the storage condition of freezer (-10~-25° C). Although the methanolic solution of the synthesized reference standard (1.0 mg/mL) could be stored under the condition of freezer (-10~-25° C) for a short time, it would take more than 6 months to decompose into 1-(4-fluorobenzyl)-1H-indazole-3-carboxylic acid.
2.3.3 Characterization of the synthesized ADB-FUBINACA through Gas chromatography-mass spectrometry (GC-MS)
The synthesized compound was freshly prepared into its methanolic solution (0.1 mg/mL), afterwards analyzed with an Agilent 7890 B series gas chromatograph in combination of an Agilent 7693 injector and an Agilent 5977B inert mass selective detector (Agilent Technologies, California, USA). The method parameters are identical with those described in 2.3.1.
2.3.4 Liquid chromatograph mass spectrometer-ion trap-time of flight (LCMS-IT-TOF)
Same methanolic solution of the synthesized compound as that for the above GC-MS analysis was diluted 100 times with methanol to a final concentration of 1.0 μ g/mL, having it analyzed into LC-MS system that consists of an LC-20AD high performance liquid chromatograph (Shimadzu, Kyoto, Japan) connected to a hybrid mass spectrometer LCMS-IT-TOF equipping with an electrospray ionization source (Shimadzu, Kyoto, Japan). An Inertsil ODS-SP column (150 mm × 4.6 mm, 5 μ m particle size, Shimadzu, Kyoto, Japan) was used to separate the compound. The mobile phase system consisted of water containing 0.05% formic acid (A) and methanol (B). For chromatographic conditions, elution was carried out with 1.0 mL/min at 35 ° C under the isocratic programming setup: 25% A at time 0 min, and being held for 40 min. The injection volume was 10 μ L. For mass spectrometric conditions, the IT-TOF mass spectrometer was operated through an electrospray ionization (ESI) nanointerface in positive mode, with the parameters being as follows: probe voltage of +4.5 kV, both the curved desolvation line (CDL) and the block heater temperature as 200 ° C, CDL voltage of +25 V, drying gas pressure of 200 kPa, and nebulizing gas flow of 1.5 L/min, Argon of the collision gas. This event was performed with the m/z scan range from 100 to 1 000.
2.3.5 Nuclear magnetic resonance spectroscopy (NMR)
10 mg of the synthesized compound was dissolved into 0.6 mL CDCl3. All NMR spectra including 1H-NMR, 13C-NMR, DEPT-135 (distortionless enhancement by polarization transfer), 1H-1H COSY (correlation spectroscopy), 1H-13C HMBC (heteronuclear multiple bond correlation), and 1H-13C HSQC (heteronuclear single quantum correlation) were recorded at 25 ° C via a Bruker Avance III 500 MHz NMR spectrometer (Bruker, Ettlingen, Germany). Chemical shifts were reported in ppm relative to CHCl3 (1H: d = 7.26) and CDCl3 (13C: d = 77.18) as internal standard.
2.3.6 High performance liquid chromatography with photo-diode array detection (HPLC-PDA)
The synthesized compound was prepared into a methanolic solution of 1.0 mg/mL. HPLC-PDA analysis was performed from a Shimadzu LC-20AD system coupling with a photo-diode array detector (Shimadzu, Kyoto, Japan). A Shim-pack HRC-ODS column (150 mm × 4.6 mm, 5 μ m particle size, Shimadzu, Kyoto, Japan) was used to separate the compound with the column chamber set at 40 ° C. The injection volume was 10 μ L, and the flow rate was 1.0 mL/min. Isocratic elution was applied through water containing 0.05% TFA (A) and methanol (B), under the programmed setup as follows: 35% A at time 0 min and being held for 35 min. The UV spectra were recorded from 190 to 400 nm, having acquired the chromatograms at 210 nm.
As shown in Fig.3, many peaks were observed in the total ion chromatogram from CG-MS analyzing the methanolic solution of the seized material. Through the obtained major-peak-shown mass spectra being analyzed one by one carefully, most of the contents of seized material cannot be found from NIST 11.0 library. The unknown peak at 19.818 min, as displayed in Fig. 3, showed major signals at m/z (relative intensity %) 109.0 (100), 253.1 (76), 338.1 (52), 309.1 (7) and 145.0 (7). As the signal of m/z 145.0 is a typical signal observed in the EI-MS spectra of synthetic cannabinoids with an indazole-3-carboxamide structure [12, 13, 14, 15], the unknown peak was suspected of indazole derivatives. By means of searching in SWGDRUG monographs library and previously published articles, the mass spectrum of ADB-FUBINACA [16, 17, 18] and that of the unknown peak were of similar signals, suggesting that the seized material was likely containing ADB-FUBINACA.
 | Fig.3 GC-EI-MS total ion chromatogram of seized material (a) and mass spectrum of the unknown peak at retention time 19.818 min (b) |
According to the recommendation of UNODC and SWGDRUG, a reliable and scientifically supported identification of seized drugs or chemicals requires appropriate analytical approach that must entail the determination of at least two uncorrelated parameters, with one of the parameters necessary to provide information on the chemical structure of the analyte. Thus, the unknown peak cannot be identified as ADB-FUBINACA unless there is a related reference standard.
The reference standard of ADB-FUBINACA was not available within the permissible time limit. Hence, the synthesis and characterization of ADB-FUBINACA had to be processed. As described in 2.3.2(2.3.3), the synthesized reference standard was characterized with appropriate analyses including GC-MS, LCMS-IT-TOF, NMR and HPLC-PDA, which were summarized as follows.
3.2.1 GC-MS
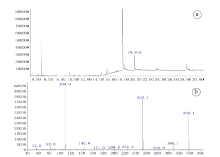
As shown in Fig.4, the major peak at 19.809 min was observed in the total ion chromatogram of the synthesized compound, demonstrating in accordance with that of ADB-FUBINACA from the earlier literatures [16, 17, 18]. The proposed fragmentation of the synthesized compound (ADB-FUBINACA?) was displayed in Fig. 4, too.
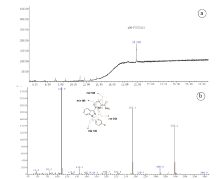 | Fig.4 GC-EI-MS total ion chromatogram of the synthesized compound (a) and mass spectrum of the major compound ADB-FUBINACA (?) at retention time 19.809 min with proposed fragmentation (b) |
3.2.2 LCMS-IT-TOF
The LCMS-IT-TOF spectral data of the synthesized compound were shown in Fig.5. The major ion at m/z 405.170 3 was identified as quasi-molecular ion corresponding to the molecular formula for the M+Na ion C21H23FN4O2Na+ (calcd. 405.169 7, error +1.48 ppm). There are two minor ions at m/z 383.186 4 and m/z 787.348 6 in the spectrum, eligible for being assigned as the M+H and 2M+Na ions, respectively. The m/z value of M+H ion is consistent with earlier published lite-rature [19].
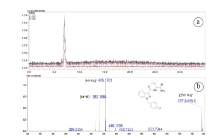 | Fig.5 LCMS-IT-TOF total ion chromatogram of the synthesized compound (a) and mass spectrum of the major compound ADB-FUBINACA (?) at retention time 7.12 min (b) |
3.2.3 NMR spectroscopy
To try best to have the structural characterization perfect and complete, NMR test was further conducted. The 1-D NMR spectra for 1H, 13C, DEPT-135 in Fig.6 and 2-D NMR spectra of COSY, HMQC, HMBC in Fig.7 were displayed, respectively.
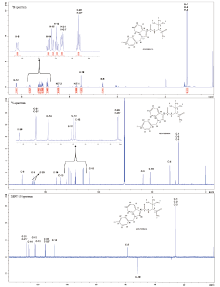 | Fig.6 1H, 13C and DEPT-135 NMR spectra of the synthesized ADB-FUBINACA (?) and the proposed proton and carbon peak assignment |
Proton and carbon peak assignment can be correlated to the structure and atom numbering as shown in Fig. 6: 1H NMR (500 MHz, CDCl3) δ = 8.22 (d, J = 8.15 Hz, 1H, H-12), 7.66 (d, J = 9.45 Hz, 1H, H-8), 7.27 (m, 1H, H-14), 7.23 (d, J = 8.45 Hz, 1H, H-15), 7.18 (m, 1H, H-13), 7.12 (m, 2H, H-21, 21’ ), 6.92 (m, 2H, H-22, 22’ ), 6.53 (s, 1H, H-7-2), 5.73 (s, 1H, H-7-1), 5.51 (s, 2H, H-19), 4.61 (d, J = 9.50 Hz, 1H, H-5), 1.09 (s, 9H, H-1, 2, 3); 13C NMR (125 MHz, CDCl3) δ = 171.9 (C-6), 161.5 (C-9), 161.4 (d, 1JCF=245.29 Hz, C-23), 139.7 (C-16), 136.1 (C-10), 130.6 (d, 4JCF=2.85 Hz, C-20), 128.0 (d, 3JCF=8.21 Hz, C-21, 21’ ), 126.0 (C-14), 122.2 (C-11), 121.9 (C-13), 121.6 (C-12), 114.8 (d, 2JCF=21.52 Hz, C-22, 22’ ), 58.6 (C-5), 52.0 (C-19), 33.7 (C-4), 25.7 (C-1, 2, 3).
3.2.4 HPLC-PDA
The methanolic solution of the synthesized compound was analyzed via HPLC to obtain the chromatographic profile. As displayed in Fig.8, the synthesized ADB-FUBINACA (?) was detected at 16.79 min as a major peak. The UV spectrum had two absorption maxima at 206 and 301 nm. Although there are several unidentified minor impurities, the purity was determined of 98.7% from HPLC-PDA with area normalization method, indicating that it is pure enough to be eligible as a reference standard.
Based on all the obtained results from the analytical methods applied above, the synthesized compound was validated as ADB-FUBINACA and can be used as reference standard. The methanolic solution of seized material and that of synthesized ADB-FUBINACA were analyzed with GC-MS under the identical conditions. Compared of the retention time and mass spectrum, the unknown compound (RT = 19.818 min) in the seized material was identified as N-(1-amino-3, 3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide or ADB-FUBINACA. In total, the whole identification took less than 25 days with such an endeavor we tried.
In this article, the ADB-FUBINCA in a seized material was identified through the identical chromatographic retention time and fragment ions compared to a chemically synthesized reference standard. A series of analytical methods were established to confirm the structure of the synthesized compound. All these methods could be applied to identify ADB-FUBINACA in forensic laboratories. As the synthetic cannabinoid market continues to evolve, it seems necessary for forensic toxicologists to expect the commercially available reference standards. However, the synthesis of reference standards offers a practical alternative for identification of emerging synthetic cannabinoids in absence of the commercially reference standards although such an endeavor is no choice but to leave on it and should be avoided as ably as possible under usual practice.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|