本文综述了2006年至今有关核磁共振技术特别是定量核磁共振技术的文献,包含对毒品特别是新型精神活性物质的研究。本文引用的文献,涉及核磁共振实验的方法开发、非特异性检测毒品、新精神活性物质等。一方面,文中介绍了核磁共振、定量核磁共振等相关技术的基本原理与方法。另一方面,本文通过国内外报道的案例,讨论了核磁共振谱仪在新精神活性物质分析检测和毒理学研究领域的应用及前景。核磁共振最大的优势在于可以对完全未知化合物的结构进行推断,并且,它是一种无损测量技术,可以用于获取多种物质的内部结构图象。当前,毒品管控面临的最大问题是:当一种精神活性物质被管控后,新的衍生物会迅速替代它,具有与管制毒品相似或更强的兴奋、致幻、麻醉等效果,这对管控制造了极大的困难。因此迫切需要有关的犯罪调查实验室通过尽量简单的样本分离和提纯步骤,从原始的粉末形式或者其它的制成消费品形式,检测并识别这类为规避法律管制而设计出的物质,但目前犯罪调查实验室面临的问题是,缴获毒品通常是包含一种或数种掺杂剂的混合物,如果不清楚混合物的组成成分信息,几乎不可能选择出一种溶剂恰好只能够溶解样本中的毒品成分。而核磁共振实验并不需要常规的萃取、纯化或衍生化等前处理步骤,对样本的纯度也没有要求,可以同时检测混合物中每一种可溶成分。二维核磁共振方法是一维谱衍生出来的新实验方法,可将化学位移、偶合常数等参数展开在二维平面上,减少了谱线的拥挤和重叠,通过提供的键键之间的偶合作用以及空间的相互作用,确定它们之间的连接关系和空间构型,有利于复杂化合物的谱图解析。定量核磁共振与核磁共振技术几乎是同时期发展起来的,其原理为根据积分信号的面积与产生共振信号的原子核的数量的关系进行定量,通过与已知浓度的标准物质信号进行比较,即可得到分析物的绝对浓度。目前,这一技术面临的主要挑战是如何提高分辨率和灵敏度。近年来,以卡西酮类、合成大麻素类为代表的新一代合成毒品在国际市场频繁出现,引发广泛关注。合成大麻素类化合物都有着芳香杂环骨架,例如吲哚、吲唑、咪唑、吡唑环等,这些骨架上可以被烷基、芳基、酰基、羰基、酯或酰胺基团所取代,产生大量的具有相同精神活性的新型衍生物。卡西酮类策划药与苯丙胺类兴奋剂的结构相似,具有强烈的中枢神经兴奋作用,通过化学修饰,也能够得到大量新精神活性物质。通过核磁共振得到特定化学环境下产生的质子信号,可以应用于对完全未知化合物结构的推断,恰好可以满足新精神活性物质的结构研究需求。此外,核磁共振谱仪与其它仪器配合使用,可以取长补短,因此文中还介绍了核磁共振谱仪与红外谱仪、气相色谱仪、液相色谱仪、质谱仪等仪器联合或比对检验的科研成果。2015年,中国增列了116种非药用类麻醉药品和精神药品列管办法,其中就包括38种合成大麻素类物质和20种卡西酮类物质。2017年初又新列管四种芬太尼类新精神活性物质。目前我国在新精神活性物质的列管机制、列管标准、列管种类、列管程序和列管时效等方面已经实现了重大突破,初步形成了对新精神活性物质的有效防控体系。但由于精神活性物质更新换代速度很快,监管总是滞后于犯罪行为的发生,越来越多的人健康受到威胁,随着这类药物滥用人数增多,其危害性逐渐被人们认知。因此应该持续加强核磁共振对新型毒品的检验方法开发,及时发现新型毒品并对其实施监管,从而预防、打击和减少毒品泛滥。
Author: LI Peng (1986—), male, master, assistant research, mainly focusing on drug detection. Email: lipeng77727@163.com
Fund: Basic Research Program Exclusive for Central Government-class Institutes of Public Welfare (No. 2016JB008)
This review is based on the literature published between 2006 and now. The methodology of NMR (nuclear magnetic resonance), in particular qNMR (quantitative NMR), was selected because of the need for the examination and quantitative analysis of controlled drugs and new psychoactive substances. NMR with external calibration is unique in its discriminative function. Experimenters may never need identical reference materials because NMR provides formidable precision and accuracy when properly handled, capable of leading towards simultaneous detection of multiple substances. New generations of cathinone and cannabinoid products are constantly emerging into market. As a result, it has become urgent for forensic laboratories to be able to promptly detect and identify synthetic cannabinoids in their original powder forms and/or other consumable products, with minimal sample preparation and clean-up steps. At present, the major challenge is the lack of both available analytical research information on these substances and reference standards, since these drugs are new to the market and forensic community and consequently have not yet been characterized. There is a critical demand for the analytical characterization of these psychoactive substances to facilitate their detection and thereby prevent their abuse. Coincidently, NMR and qNMR provide the capability to meet the demand.
NMR was conceived as a quantitative technique from the very beginning: quantitative NMR (qNMR) is almost as old as NMR itself. Indeed, quantitative analysis has been associated with NMR spectroscopy as it is based on the proportional relationship of the integrated signal area and the number of nuclei generating the resonance. The absolute concentration of an analyte can be determined by comparison of the integrated intensity of the resonances of the sample to a standard of known concentration[1]. Fig.1 illustrates the current landscape of quantitative NMR. One problem forensic laboratories face when analyzing submitted drug evidence is that the samples are often mixtures likely to contain one or more of several cutting agents. Without knowledge of the mixture components, it is impossible to select a solvent that will (ideally) only dissolve the drug of interest for interpretation. NMR requires neither common pre-processing steps such as extraction, purification or derivatization, nor pure samples. Still, as it is non-selective, NMR, unlike other methods, can simultaneously detect all compounds within a mixture in a single experiment provided they are soluble in the solvent and have the nuclei being probed in sufficient quantity for detection and assignment. Additionally, because of its inherent quantitative property, NMR can attain high precision when following specific guidelines discussed by Griffiths and Irving[2]. In consequence, NMR affords a favorable choice for forensic chemists and toxicologists to identify illicit drugs.
Given this background, the goal of this review is not to provide an extensive description of the application of the field of NMR, but rather to offer a modest and personal viewpoint on the present status and future prospects of this exciting research field.
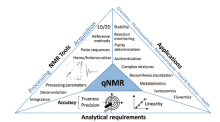 | Fig.1 The landscape of quantitative NMR (qNMR) in 2017[1] |
New combinations of potentially lethal drugs are being invented nearly daily, producing adverse effects such as anxiety attacks, vomiting and psychotic episodes when administered. The international drug control authorities are perplexed at the proliferation of these substances. This predicament is mainly profit-driven by drug traffickers to circumvent the highly diverse legislation in a multitude of different countries which are only slowly and passively adapting their national narcotic laws. For instance, new synthetic cannabinoids have been dramatically introduced into the illicit drugs market since 2012[3] (Fig.2), leading to an estimated 14 ~ 30 times higher risk of medical emergency treatment compared to the use of cannabis [4, 5, 6]. These new drugs have been called “ legal highs” , “ bath salts” and “ spice” amongst other names, but the name preferred by international agencies such as the United Nations Office on Drugs and Crime (UNODC) is “ New Psychoactive Substances” (NPS).
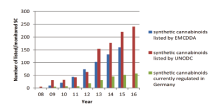 | Fig.2 Number of synthetic cannabinoids (SC) reported in publications by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), UNODC and German Betä ubungsmittelgesetz (BtmG)[4] |
Detection of these drugs, synthesized to mimic existing illegal substances, demonstrates an achievement in technology[7]. Among these new psychoactive substances, “ synthetic cathinones” burst into the market at an explosive rate which shows no signs of slowing. Synthetic cathinones are β -keto phenethylamines, structural analogs of cathinone, a psychoactive stimulant found naturally in the khat plant (Catha edulis)[8, 9, 10, 11, 12, 13, 14, 15].
Additional information about the pharmacological action of several pyrrolidinophenones (pyrrolidinovalero-phenone, pyrovalerone, 3, 4-methylenedioxypyrovalerone, 3, 4-methy-lenedioxypyrrolidinopropiophenone, and 3, 4-methylenedioxypyrrolidino-butyrophenone) was recently published[16, 17], showing that all pyrrolidi-nophenones were potent norepinephrine and dopamine inhibitors.
A study of the street drug mephedrone seized in South Wales over two years showed a decrease in sample purity after it was banned in the UK, with the most common cutting agents being sodium monoglutamate, sucrose and creatine[18, 19]. Studies on the composition of “ legal highs” marketed in Portugal revealed that products were usually mixtures of synthetic cathinones and several other ingredients such as glucose, lactose and caffeine [20, 21]. Justin et al[22] used the complementary techniques of IR and NMR spectroscopy to initially identify the contents of “ Raving Dragon Novelty Bath Salts” , the so-called first generation of this type of drug, and the second generation, “ Raving Dragon Voodoo Dust” . The NMR-generated structures agreed with the IR data and allowed the confirmation of the first-generation product, the Raving Dragon Novelty Bath Salts as methylone and of the second-generation product, Raving Dragon Voodoo Dust, as pentedrone. Khaled et al[23] demonstrated, for the first time, the combination of HPLC-UV with amperometric detection (HPLC-AD) for the qualitative and quantitative analysis of 4-methylmethcathinone (4-MMC, Mephedrone) and 4-methylethcathinone (4-MEC) (fully characterised by 1H-NMR, 13C-NMR, given the purity> 99.5% in both cases) using either a commercially available impinging jet (LC-FC-A) or custom-made iCell channel (LC-FC-B) flow-cell system incorporating embedded graphite screen-printed macroelectrodes (Fig.3). The protocol offered a cost-effective, reproducible and reliable sensor platform for the simultaneous HPLC-UV and amperometric detection of the target analytes. The two systems had similar limits of detection, in terms of amperometric detection (LC-FC-A: 14.66 μ g/mL and 9.35 μ g/mL, LC-FC-B: 57.92 μ g/mL and 26.91 μ g/mL), to the previously reported oxidative electrochemical protocol (39.8 μ g/mL and 84.2 μ g/mL), for the two synthetic cathinones, prevalent on the recreational drugs market.
 | Fig.3 FC-A impinging jet flow cell (a: closed; b: open) and FC-B iCell channel flow cell (c: closed; d:open), with (e) the flow diagram of HPLC-AD systems (LC-FC-A and LC-FC-B)[23] |
In 2015, the Australian Border Force (ABF) seized a kind of viscous, light-red liquid[24]. By a combination of NMR, GC-MS and infrared spectroscopy, the unknown substance was identified as N-tert.-butoxycarbonyl-MDMA (t-BOC-MDMA). Through exposure of the t-BOC-MDMA to a simulated gastric juice (pH 1.5) to validate the existence of a pro-drug and formation of MDMA, the -O-CH2-O- resonance corresponding to t-BOC-MDMA was shown by NMR to decrease in size while the -O-CH2-O- resonance from methylenedioxyregion of the proton NMR spectrum of the MDMA product increased during the same period. At t=305 min under pH 1.5 and T=37℃, the majority of the t-BOC-MDMA had converted to MDMA. Given this biochemical state does not naturally occur in a human stomach during physiological digestion, it seems conceivable that ingestion of the t-BOC-MDMA might result in a slow release of MDMA formation in vivo. It is believed that this is the first time in the world that MDMA, concealed as the tertiary-butoxycarbonyl derivative, has ever been detected by law enforcement agencies. Folker et al[25] reported a seizure of the same compound in 2016 by police in North-Rhine Westphalia, Germany.
Jacek et al[26] identified three novel hydrochloride salts of cathinones: 2-(pyrrolidin-1-yl)-1-(5, 6, 7, 8-tetrahydro-naphthalen-2-yl)pentan-1-one (TH-PVP), 2-(methyl-amino)-1-(2-methylphenyl)-1-pro-panone (2-MMC) and 1-(4-chlorophenyl)-2-(methylamino)propan-1-one (4-CMC). Their properties were examined through combinations of GC-MS, IR, NMR, electronic absorption spectroscopy and single crystal X-ray diffraction. In addition, characteristic reactions for the identification of these compounds were devised to focus on the carbonyl group, the most distinct part of cathinones in comparison with amphetamine-based compounds. Tetraphosphorus-decasulfide (P4S10), Davy, Heimgartner or Lawesson’ s reagents (LR) have been reported to convert carbonyls into thiocarbonyl compounds. However, this type of transformation has only been found to have limited applications for the synthesis of simple thioketones because they were relatively unstable at room temperature. So, P4S10 and LR were selected by the authors for their thionation reactions due to the low cost of the starting materials and simplicity of the synthesis. Complex mixtures of products were obtained, resulting from the reaction of both the carbonyl and amine groups with the thionation reagents. Their reactivity was evidenced by the complicated 31P NMR spectra with four intense signals at 93.9, 90.9, 83.5 and 81.4 ppm, which were characteristic for compounds owning N-P(=S)S2fragments[27]. Cemal et al[28] reported similar type of reactivity leading to N-phosphorylated products. Shima et al[29] detected 1-phenyl-2-(pyrrolidin-1-yl)octan-1-one (PV9) and 16 of its metabolites, including diastereomers and conjugates in PV9 users’ urine using newly synthesized authentic standards and/or based on their mass spectra and 1H-NMR. Interestingly, it was suggested that the main metabolic pathways of α -pyrrolidinophenones depend on alkyl chain length. The authors concluded that their fi ndings will contribute to the establishment of a reliable analytical procedure for proving the intake of newly encountered designer drugs, as well as to predict their metabolic pathways. Similar research has been done with α -pyrrolidino-butiophenone (α -PBP)[30, 31, 32]. Koutaro et al[33, 34] exhibited a fatal case of PV9 poisoning where a PV9 metabolite (via reduction of keto group) was identifi ed based on LC-MS/MS data. Kayoko et al[35] also narrated detections of 1-Phenyl-2-(pyrrolidin-1-yl)octan-1-ol and an oxidized metabolite of PV9 in human blood.
Many other newly distributed designer drugs have been reported[36, 37, 38, 39, 40, 41, 42, 43].
The composition of synthetic cannabinoids is ever-changing[44]. New generations of these so-called “ Spice” products are constantly being released into the illicit drugs market, continuing to cause harm. As a result, it has become urgent for forensic laboratories to be able to promptly detect and identify synthetic cannabinoids in their original powder form and other consumable products, with minimal steps of sample preparation and cleanup. Most newly emerging synthetic cannabinoids exhibit an aromatic heterocyclic skeleton, for example indole, indazole, imidazole, or pyrazole rings, which can be substituted by diverse types of alkyl, aryl, acyl, ester, carbonyl, or carboxamide groups and lead to a large variety of new derivatives continuing to emerge on the synthetic drug market[45, 46, 47, 48, 49, 50].
In China, 116 narcotic drugs and psychotropic substances for non-medical use were placed under control on 1st October 2015, including 40 synthetic cannabinoids in addition to the 12 substances first regulated in 2013. Among the samples seized from a clandestine laboratory uncovered in Hubei province, seven synthetic cannabinoids and five substituted phenethylamine derivatives have been identified[51, 52, 53]. NMR has been extensively used to derive the structures of purified synthetic cannabinoids. With NMR confirmation, the position of the substitution group on an aromatic ring can be determined (when compared to standard mass spectrum) as each proton in a unique chemical environment produces a unique signal that remains the same even in a herbal mixture (Fig.4). Slightly modified chemicals like JWH-073 flooded into the newly-born market for this kind of drug with a myriad of similar plant-based second-generation ‘ ‘ Spice-like’ ’ products misleadingly sold as incenses, meditation potpourris, bath additives and related others. Frequently, a compound had hardly been banned by the authorities when new structures/products were introduced even faster. NMR spectroscopy has long been used in forensic laboratories for elucidating the structures of novel compounds and is now much prized for the quantification of drugs and adulterants in potentially illegal products of abuse[54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87].
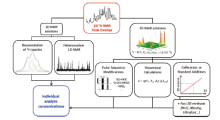 | Fig.4 NMR strategies capable of disentangling overlapped 1D NMR peaks to yield accurate individual structures |
Jankovics et al[88] identified and characterized two new potential cannabimimetic molecules by several analytical techniques. The substances were seized in the form of pure powders by the customs office in Hungary. LC-MS/MS and UV spectra of JWH-018 and JWH-122 were used for their structure elucidation, which was confirmed by 1H-NMR and 13C-NMR. Taking into account the circumstances of their seizure, both powder samples might have been intended to be used as designer drugs in their pure form or as ingredients of ‘ ‘ smart products’ ’ . Langer et al[89] identified eight different synthetic cannabinoids by GC-MS: THJ-018, THJ-2201, MAB-CHMINACA, 5F-ADB, 5Cl-AKB48 (syn.:5C-AKB48), 4-pentenyl-AKB48, MDMB-CHMICA and 5F-AB-PINACA using JWH-018 as the GC-MS internal standard and the corresponding response factors to calculate the total amount of all synthetic cannabinoids in the commercial smoking mixtures. NMR and other techniques were used to fully characterize the compounds. The content of synthetic cannabinoids in the investigated products ranged from 23 to 120 mg/g (average: 57 mg/g), while the individual compounds ranging from 1 to 120 mg/g. Langer also carried out a full iterative band-shape analysis of the indazole and naphthalene proton signals. The metabolism of THJ-018 and THJ-2201 in human hepatocytes has already been examined[90] and compared with the benzimidazole analogue of AM-2201 (=FUBIMINA)[91, 92]. THJ-2201, an indazole analogue of AM-2201, was isolated from herbal mixture and was fully described in the literature. In an analysis of 104 chemical- and herbal- type products, THJ-2201 was one of eight newly distributed synthetic cannabinoids[91]. MAB-CHMINACA, detected in herbal blend, is also known as ADB-CHMINACA whose mass spectra were first described by Wurita et al[82] after it had been encountered in herbal mixtures. NMR data of 5F-ADB was obtained from a CD3OD solution[50]. The 13C chemical shifts in CD3OD were generally higher than the literature values in DMSO-d6 by 1.5 ppm with a standard deviation of 0.5 ppm. Synthetic cannabinoids with a 5-chloropentyl chain moiety have been isolated from herbal blends before, albeit generally only in minor amounts compared to the 5-fluorinated main product[89, 93] among which the synthetic cannabinoid was found as the major ingredient at a concentration of 76 mg/g accompanied only by a hitherto unknown trace. The 5-fluoro analogue 5F-AKB48 showed a high affinity to the CB1 receptor with a Ki of 0.87 nM[94]. MDMB-CHMICA, characterized and quantified in one herbal mixture, was reported in 2015 and cases of intoxication and death attributed to its consumption have been published[95, 96]. 5F-AB-PINACA was fully characterized in 2015 when its metabolic profile in human hepatocytes[97] and pharmacological characterization in rats[98] were reported.
A sample of “ 4-methylpentedrone(4-MPD)” was obtained, along with information including incorrect structures and nonexistent CAS numbers, and analyzed by GC-MS, NMR, and liquid chromatography-electrospray ionization (LC-EIS)[99]. The molecular mass obtained from GC-MS indicated that this vendor sample, labeled 4-MPD, might contain an additional -CH2. Hence, the 1H, 13C and, 13C DEPT NMR spectra for 2-(ethylamino)-1-(4-methylphenyl)pentan-1-one (4-MEAP), the above vendor sample and 1-(4-methylphenyl)-2-(propyl-amino)butan-1-one (4-MPB) were stacked to make a comparison of the NMR data along with the unique fragmentation patterns produced from these molecules. The spectra for the vendor sample and 4-MEAP were nearly identical, with the ppm shift values differing by < 0.2 ppm overall (Fig.5). Thus, the sample was finally identified as 2-(ethylamino)-1-(4-methylphenyl)-1-pentanone (4-MEAP), not 4-MPD or 4-MPB.
 | Fig.5 The 1H (left), 13C (center) and DEPT 13C NMR spectra (right) for the 4-MEAP, the vendor sample and 4-MPB[99] |
Karen et al[100] first reported an aryl sulphonamide cannabimimetic in seized plant materials in Western Australia. The identification of 8-quinolinyl-4-methyl-3-(1-piperidinylsulfonyl)benzoate (QMPSB) in methanol extracts of seized plant materials was shown to be hampered by almost complete transesterification to its methyl ester methyl 4-methyl-3-(1-piperidinylsulfonyl) benzoate (MMPSB), even at ambient temperatures. The presence of hydroxyquinoline in a seized plant material extract is an indicator that transesterification of a quinolinyl ester may have occurred. Edmunds et al[101] described the isolation and characterization of a new synthetic cannabinoid. The mass spectrum highlighted structural similarities to JWH-081, though the spectrum showed no match in available libraries. Finally, the use of NMR spectroscopy plus high resolution LC/MS allowed for structural elucidation and identification of the unknown as (1-(cyclohexylmethyl)-1H-indol-3-yl) (4-methoxynap-thalen-1-yl)methanone according to the key signals in the 1H NMR spectrum (Fig.6) to include a downfield doublet at 3.91 ppm due to the N-bound methylene protons.
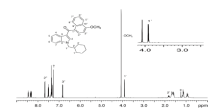 | Fig.6 1H NMR spectrum for (1-(cyclohexylmethyl)- 1H-indol-3-yl)(4-methoxynaphthalen-1-yl)- methanone[101] |
Analytical challenges still remain in the detection of these synthetic cannabinoids due to the difficulty in obtaining all possible standards and in deconvoluting the interference signals from the background matrix. Dunne and co-workers[102] developed an extraction procedure compatible with direct NMR quantification of synthetic cannabinoids in herbal smoking blends. Extraction media, time and efficiency were tested for different carrier materials containing representative synthetic cannabinoids. The developed protocol utilizes a 30 min extraction in d4-methanol in the presence of an internal standard, allowing direct quantitation of the extract by NMR. The accuracy of the developed method was tested with in-house prepared herbal smoking blends. The results showed deviations less than 0.2 % from the actual content, proving that the method is sufficiently accurate for the relevant quantifications. Obtained results showed a variation in cannabinoid contents from 1.5 % (w/w) for mixtures containing MDMB-CHMICA to over 5 % (w/w) for mixtures containing 5F-AKB-48. This is an extremely effective advantage, since reference materials of new synthetic cannabinoids are often difficult to obtain when the substance is first introduced into the illicit drug market. Marino et al[103] designed their NMR sample preparation as a simplified protocol to dramatically reduce both the time and sample size that were needed to positively identify cannabinoids in herbal products. The combination of rapid direct analysis in real time-mass spectrometry (DART-MS) and NMR can provide concrete cannabinoid structural information with no ambiguity, permitting this strategy to be a useful alternative, or complement, to the conventional GC-MS and LC-MS choices. According to their study, the four-minute 32-NMR scans generated a S/N of 4 to 1 for as little as 50 μ g (slightly above LOD) of a cannabinoid sample with successful identification. Fowler et al[104] directly extracted synthetic cannabinoids with NMR solvent without any conventional lengthy isolation, purifi cation or chromatographic separations. 1H NMR[105] and proton correlation spectroscopy (COSY, HMQC and HMBC)[106] were successfully employed to generate molecular fi ngerprints in herbal extracts, taking advantage of the spectroscopic separation power of NMR spectroscopy. The identification and quantification steps can be completed within an hour from sample-in to answers-out (Fig.7).
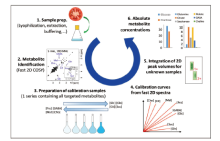 | Fig.7 Analytical workflow illustrating the quantitative method capable of determining absolute metabolite concentrations[106] |
New synthetic opioids and the sedative present a serious problem for public health due to their potency and risk of fatal intoxication[107, 108, 109, 110]. “ Krokodil” , a homemade injectable mixture being used as a cheap substitute for heroin, is a light brown liquid that may cause the skin to become iridescent and discolored, and further lead to desquamation if injected without previous purification. Repeated or regular use may turn the skin around the injection site scaly and rough, like crocodile skin. Neves et al[111] devised a thin-layer chromatography (TLC) profile, UV/Vis, 1H-NMR and FTIR spectrum methods to screen and quantify desomorphine and codeine in ‘ ‘ krokodil’ ’ samples to show further information about the contaminants present in “ krokodil” . The synthesis was carried out as described in a previous paper[112], where crude “ krokodil” appeared as a yellow to light brown solution due to the presence of iodine[113]. Treatment of crude “ krokodil” with a strong base assured the presence of morphinans as free bases, which were subsequently extracted with ethyl acetate, an organic solvent of intermediate polarity. The qualitative analysis of the samples also showed the presence of other two morphinans (i.e. dihydromorphine-3, 6-dideoxy and morphinan- 4, 5-epoxy-3-ol) due to the highly reductive environment. The authors contacted users to understand the synthesis process and to know all the social and legal issues that lead a person to abuse “ krokodil” . A new synthetic analogue of fentanyl, N-phenyl-N-[1-(2-phenethyl)piperidin-4-yl]prop-2-enamide (acrylfentanyl), was identified in powder from a seized capsule found at a forensic psychiatric ward in Denmark[114]. The proportion of deaths due to illicit drug overdose involving fentanyls has grown to exceed 50% in some parts of Canada[115] during the recent few years. Since 2012, 12 deaths in Russia[116] and more than 50 deaths in the US have been associated with acetylfentanyl[117, 118, 119]. Other fentanyl analogues, such as butyrfentanyl, 3-methylfentanyl and furanylfentanyl, have also been linked to serious adverse events[120, 121, 122].
Novel methods were introduced in this review for the detection of drugs based on NMR in combination with diverse analytical techniques. The major challenges for the instrumentation - resolution and sensitivity - are of course not new and not specific to qNMR. Meanwhile, the main obstacle for drug analysis is the lack of both the analytical research information available on those illicit drugs and their reference standards since they are new to the market and consequently not yet been characterized[123, 124, 125]. Thus, there is a critical need for the analytical characterization of these new psychoactive substances to facilitate their detection[126].
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|


