作者简介: 黄 威,副研究员,博士,研究方向为声像资料。 E-mail: huangwei@cifs.gov.cn
纳米材料具有晶粒微小,表面积大,扩散率高,相互作用强烈等传统粉末不具备的特殊性质,表现出独特的物理化学性质,受到了国内外法庭科学专家的青睐,逐渐成为国内外手印检验专业研究的热点。目前研究比较集中的纳米材料主要有镉、铕等半导体纳米材料,金、银、锌、铁、钛等金属纳米粒子,上述材料在一定程度上具有毒性,以二氧化硅作为基本材料的纳米粒子,是一种无毒、无味、无污染的无机非金属材料,具有量子尺寸效应、量子隧道效应和特殊的光电特性,具有高强、高韧、稳定性好等优势。当纳米二氧化硅与水溶液接触时,表面不饱和的残键得到修复,与水分子之间表现出很强的吸附能力,同时也使纳米二氧化硅在油性介质中极易团聚,分散性和稳定性较差,若将纳米二氧化硅粒子应用于手印的刷显过程,必须对其进行表面亲油改性,提高显现手印的能力。本文在归纳和总结半导体及金属纳米材料显现手印的发展历程和最新进展基础之上,选择具有表面易改特性的二氧化硅纳米材料作为基础材料,提出了合成新型亲油性二氧化硅纳米复合材料的技术方法,并对新型纳米复合材料显现非渗透性客体上潜在手印的能力与传统粉末法进行了比较考察。首先,采用Stober溶胶-凝胶法制备二氧化硅纳米颗粒,用十八烷基三氯硅烷(octadecyltrichlorosilane,OTS)进行表面亲油性改性。十八烷基三氯硅烷与硅球表面的硅羟基发生缩合反应,脱去氯化氢,最终生成的二氧化硅表面的Si-R和Si-OH基团共存,在较高的十八烷基三氯硅烷浓度下,二氧化硅表面的羟基几乎被全部取代,从而生成表面只有Si-R基团的亲油性纳米二氧化硅复合材料。通过调控十八烷基三氯硅烷与纳米二氧化硅的用量比,改变二氧化硅纳米球表面的Si-R和Si-OH的基团比,得到不同改性程度的二氧化硅纳米复合材料。再利用扫描电镜(SEM)、傅里叶变换红外光谱检测(FT-IR)、X射线光电子能谱测试(XPS)、X射线衍射测试(XRD)对改性纳米粒子进行表征。结果表明,合成的新型二氧化硅纳米粒子亲油性能良好,形态均一,稳定性强。然后通过由十八烷基三氯硅烷修饰的纳米二氧化硅粉末处理非渗透性客体表面潜在油汗混合手印,对影响粉末法显现效果的二氧化硅纳米颗粒的粒径和二氧化硅改性程度进行调整,通过综合考察手印表面有无滞粉、粘粉以及纹线的连续性等因素最终确定二氧化硅与十八烷基三氯硅烷的质量配比为10׃1,粒径为700 nm的二氧化硅粉末显现效果较好。将十八烷基三氯硅烷改性的新型亲油性二氧化硅纳米粉末与常用的金粉、银粉和黑色磁性粉进行手印显现比对实验,在自然光下观察不同粉末显现非渗透性客体(玻璃、陶瓷、黑色硬塑料)表面潜在油汗混合手印效果,新型纳米粉末显现出的手印图像纹线清晰连贯,背景反差好,细节特征清晰。本研究设计合成的纳米二氧化硅粉末在有机相中具有一定的分散性,解决了纳米粒子在有机相中的软团聚现象,增强了与手印残留物的结合能力;颗粒的粒径处于纳米级,提高了与手印残留物的粘附性;由于这种复合材料选择性高,仅与手印纹线上的油脂发生粘附作用,可以减小手印图像的背景干扰,增强对比度,可以用于显现非渗透性客体表面的潜在油汗混合手印。
Author: HUANG Wei, Ph.D., associate research fellow in photograph and video examination. E-mail: huangwei@cifs.gov.cn
Corresponding Author: LI Xiaojun, M.S., assistant research fellow in fingerprint examination. E-mail: lixiaojun@cifs.gov.cn
Motivated by the demand for the development of latent fingerprint with nanotechnology, a new type of nanometer-sized amphiphilic silica particles was synthesized by sol-gel method and modified using tetraethyl orthosilicate (TEOS) and octadecyltrichlorosilane (OTS). The prepared particles were characterized by various methods to show that their structure and properties were adequate for fingerprint development in the forensic science field. When compared to other silica particles, the modified powder appeared highly lipophilic and monodisperse. The effects of synthesis conditions and size of silica sphere were also discussed. The high-quality fingerprints on smooth nonporous objects were developed using the new type of particles, indicating that a new sensitive latent fingerprint developing assay was obtained. The prepared particle exhibits such attractive features as time saving, simple procedures, convenient operation and reasonable price.
For decades, fingerprint has remained one of the most important forensic evidence because it builds a direct link between a person and a crime scene or a related object. When unprotected hands touch a surface or an object, the ridge pattern on the finger tip of the individual, or “ fingerprint” , is transferred to the surface through the deposition of sebaceous and eccrine secretions. Normally, fingerprints are not visible and are called “ latent fingerprint” , requiring techniques to make it visualized. Large numbers of techniques[1, 2, 3, 4]have been applied in fingerprint development area. They are mainly characterized by developing efficiency, based on the composition of the materials, the surface of the object (porousness, wetness), the age of fingerprint, and others. The available techniques involve various approaches either using dusting agent that adheres to the “ sticky” secretions deposited on the object’ s surface[5, 6, 7, 8], or chemical developer that generates chemical interaction with the deposited materials on the fingerprint to produce visual color[9, 10, 11]. For those agent techniques, powder composites are usually preferred due to their detectable sensitivity and their ability to obtain the nature of the pattern or the color of the support on which the fingerprints deposit. Numerous powders with different characteristics such as fluorescent[12], non-fluorescent[13], magnetic[14], non-magnetic and colored[15], are commercially available. During the past 20 years, nanoparticles or nanostructured materials have shown to be the promising alternative to conventional techniques. Nanoparticles have size-dependent properties due to their nano-size range, difference from atomic size particles or bulk material. Generally, the nanostructured ones driven by golden nanoparticles[16, 17] can be distinguished from quantum dots and silica nanoparticles. Each of them possesses its advantages and drawbacks in terms of efficiency and sensitivity according to the nature of the object’ s surface, the latent materials and other conditions. Though semiconductor quantum dots (QDs e.g., CdSe, CdTe, ZnS, HgTe, InP, GaAs or InAs) have become a hot field of forensic science research over the last decade [18, 19, 20, 21, 22, 23], silica nanoparticles differ from QDs in a sense that they are optically inert on their own. Meanwhile, one of its advantages is that the reactions are conducted at low temperature, permitting organic and inorganic species to coexist within the same matrix.
Among the various modes of nanoparticles, materials based on silica (SiO2) obtained by the sol-gel process appear to be an excellent choice for function modification in chemical development area[24, 25]. These particles have better surface area, higher chemical and thermal stability, and active surfaces due to silanol groups (Si-OH), providing further potential for further development[26, 27]. Silica matrix can be modified in several ways, one of which is post-modifications of a silica matrix by covalent bonding of the carboxyl groups of lipophilic or hydrophilic[28]. Amphiphilic silica nanoparticles are very important for forensic fingerprint detection since the ridge deposits often consist of organic molecules, such as long carbon chain fatty acids, amino acids as well as inorganic salts and a multitude of unknown contaminants[29]. As such, this method can be used to obtain modified silica spheres and produce a new class of building blocks. It could be interesting to study how this nanoparticle develops fingerprints, and then eventually improves the detecting efficiency of latent fingerprints[30, 31].
In this article, a simple and cost-effective self-assembly approach is presented to prepare silica nanoparticle using tetraethyl orthosilicate (TEOS) and octadecyltrichlorosilane (OTS). Several work-up methods were utilized to characterize the new nanoparticles. The effects of different TEOS:OTS weight ratios and particle diameters on detecting latent fingerprints were tested.
TEOS and OTS were purchased from Alfa Aesar (Shanghai, China). Ethanol, ammonia and n-hexane were from Beijing Chemical Reagents Company (Beijing, China). All the chemicals were of analytical grade and were used as received without further purification. Deionized water was used throughout the research work.
Monodisperse silicon dioxide spherical particles were obtained in situ by mixing TEOS with ethanol and deionized water. Ammonia was used as a catalyst during the hydrolysis and condensation processes. OTS was used as a surface modifier to obtain amphiphilic silica particles. More specifically, the following procedure was used to obtain amphiphilic silica nanoparticle. In the first step, ethanol, deionized water and ammonia (25%) were added into a three-necked flask in sequence, and stirred for about 30 min at room temperature. Different quantities of TEOS were then added (0.5, 1 and 2 g) to obtain particles with different diameters. The solution was stirred for 8 hours and then washed 5 times using high-speed centrifugation at 5500 r/min (TDL-5-A, Shanghai Anting Scientific Instrument Factory, Shanghai, China). Finally, the monodisperse hydrophilic silicon dioxide spherical particles were dried in a vacuum oven (202-l, Shanghai Yiheng Instruments Co., Ltd., Shanghai, China) for 24 hours. Particles with three average diameters of 250, 550 and 700 nm were obtained. In the second step, the monodisperse hydrophilic silicon dioxide spherical particles were modified by reducing 1 g of silica powder in 100 mL n-hexane. The silica particles were dispersed 30 min using an ultrasonic oscillator. 0.01, 0.02, 0.1 and 0.5 g of OTS were added to obtain particles with the following SiO2: OTS weight ratios: 100:1, 50:1, 10:1 and 2:1. The solution was stirred for 12 hours. The amphiphilic silica nanoparticles were washed twice using high-speed centrifugation at 5500 r/min and dried in a vacuum oven for 24 hours.
The morphology of the amphiphilic silica nanoparticles was examined by field-emission gun scanning electron microscope (FEG-SEM) of a Hitachi S-4800 type (Tokyo, Japan) equipped with a high-resolution secondary electron detector (in lens detector), operated at 10.0 kV and a point-to-point resolution of 2 nm. The optical properties of the silica nanoparticles were characterized using Fourier transform infra-red spectrometer (Perkin-Elmer Corporation, Waltham, USA). Spectra were recorded using the 514.5 nm line of a helium-neon laser at a power of 150 W and spectral resolution of 1 cm-1. The X-ray photo electron spectrum (XPS) of the amphiphilic silica nanoparticles was obtained using an ESCALAB250Xi (Thermo Fisher Scientific Ltd., Waltham, USA) hemispherical electron analyzer, and an aluminum anode as the X-ray source. The X-ray source was operated at 12 keV and 15 mA. Binding energies set at 103.4 eV were corrected using the reference Si2p line from silica, X-ray powder diffraction (XRD) analysis was performed on a Rigaku D/max-2500 diffractometer (Rigaku Corporation, Tokyo, Japan) equipped with Cu Kα radiation (λ =1.54178Å ) operating in continuous scan mode at 2° min-1. The measurements were collected at room temperature with a scan range between 3° and 60° .
The performance of amphiphilic silica nanoparticle powder for the detection of latent finger impressions was measured using a series of 300 latent fingerprints. The fingerprints were obtained from 10 donors, who were asked to wash their hands thoroughly with soap. Each one of the 10 fingers from each donor was gently rubbed across the donor’ s forehead and deposited on clean microscopy glass slides. This process was repeated 3 times for each donor, resulting in 3 sets of 100 latent prints. The sample sets were kept at room condition for 1, 3 and 7 days before processed with the silica nanoparticle powder.
Detection of the latent fingerprint was performed using the different particles obtained as described above (i.e., particles with different diameters and different SiO2: OTS weight ratios). Brushing was done using squirrel hair fingerprint brushes. Fingerprint images were captured using a DCS4 fingerprint enhancement system (Foster & Freeman Ltd., Evesham, UK).
During the preparation of the monodisperse hydrophilic silica nanoparticles, TEOS was hydrolyzed to form the corresponding hydroxylated products and alcohol; meanwhile, the condensation reaction between silicic acid itself and TEOS occurred. The reaction equations show as below:
Si(OR)n+xH2O→ Si(OH)x(OR)n-x + xROH
-Si-OR + HO-Si-→ Si-O-Si + ROH
-Si-OH + HO-Si-→ Si-O-Si + H2O
During the modification step of the procedure, OTS reacted with the silanols from the surface of the silicon spheres, resulting in the loss of Cl- and H+; eventually Si-R and Si-OH groups coexisting at the surface of the silica particles. If the concentration of the OTS was high enough, the hydroxyl groups on the surface of the nanoparticles were almost all replaced, causing lipophilic nano-SiO2 composite particles with Si-R groups on their surfaces. By adjusting the weight ratios of OTS and TEOS, the ratio of Si-R and Si-OH groups on the silica nanoparticle could be changed, therefore, different levels of modified amphiphilic silica particles were offered.
3.2.1 FEG-SEM Images
The FEG-SEM images of the hydrophilic silica material before modification showed that the particles were highly soluble in water, but not in hexane (Fig.1). This implied that hydrophilic silica particles would not dissolve in the amino acid component of latent fingerprint residue. This was the main reason why hydrophilic silica particles were not appropriate for detecting latent fingerprints. On the other hand, silicon spheres, modified by OTS, showed excellent solubility in hexane. The solubility appeared to increase with the weight ratio (WR) of SiO2 to OTS (Fig.2). According to the results, the bigger the weight ratio of SiO2 to OTS, the better the nanoparticles adhere to each other. The weight ratio of SiO2 to OTS became the key factor to affect the ability of amphiphilic silica nanoparticles to detect latent fingerprints.
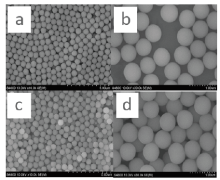
 | Fig.2 SEM images of the amphiphilic silica particles in hexane bath with the weight ratio of SiO2: OTS at 2:1 (a), 10:1 (b), 50:1 (c) and 100:1 (d), respectively |
3.2.2 FTIR Spectra for Amphiphilic Silica Nanoparticle
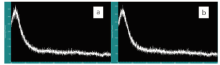
Fourier transform infrared spectra provided important corresponding data for the characterization of the structure of the nanoparticles in the amphiphilic silica material by offering information on the microstructure of the material. The spectra of silica sphere before modification could be seen in Fig.3a. In Fig.3a, three peaks at 797, 1100 and 1400 cm-1 were observed. The peak at 1100 cm-1 was the antisymmetric stretching vibration, belonging to Si-O-Si, and the peak at 1400 cm-1 was the stretching vibration of C-O. These two peaks showed that some Si-C groups were present on the surface of the silica sphere before modification. The peaks at 935 and 3414 cm-1 reflected the bending vibration of Si-OH and the stretching vibration of -O-H. On Fig.3b~3d, the peaks near 2921 and 2852 cm-1 reflected the antisymmetric stretching vibration arising from -CH2- alkyl chain, which showed that alkyl groups had successfully replaced some of the hydroxyl groups on the surface of silica spheres. The peak at 1469 cm-1 reflecting the asymmetric bending vibration of -C-H-, also explained above view. The peak near 1632 cm-1 might be caused by the absorbed water during the particlesʼ preservation process. Fig.3a-3d showed that the intensity of the characteristic absorption peaks gradually increased with the rise of the weight ratios. It also meant the function of adherence could be strengthened by the increase of the weight ratios.
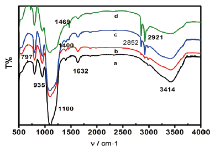 | Fig.3 FTIR spectra of unmodified silica sphere (a), amphiphilic silica particle modified by the weight ratio of SiO2׃ OTS at 100׃1 (b), 50׃1 (c) and 10׃1 (d) |
3.2.3 X-ray Photoelectron Spectroscopy
XPS analysis was conducted in order to obtain information about the surface and the feature of the chemical bonds. Fig.4 showed the XPS binding energy (eV) values of C1s, O1s and Si2p were acquired for the silica nanoparticles. The binding energy (B.E.) value of C1s at 284.7 eV was assigned to carbon bonded atoms in polyaromatic structures in graphite (B.E.¼ 284.6 eV), or carbon presented in alcohol[32, 33, 34, 35]. As a consequence, the binding energy value of O1s at 532.8 eV was assigned to carbonyl oxygen atoms in esters, and anhydrides and oxygen atoms in hydroxyl groups. This implied that the modified silica material was ideal for further efficient chemical modifications.
 | Fig.4 XPS images of unmodified silica sphere (a), amphiphilic silica particle modified by the weight ratio of SiO2: OTS at 50:1 (b), 10:1 (c) |
3.2.4 X-ray Powder Diffraction
To show that the silica material had a disordered structure, the XRD patterns of the unmodified silica sphere and amphiphilic silica nanoparticle were obtained and compared (Fig.5). It showed that both the unmodified material and amphiphilic silica presented an amorphous structure, which could be inferred from the absence of peak reflection in the XRD diffractogram. Based on these results, it was safe to say that neither of the particle types had periodicity, but both were disordered.
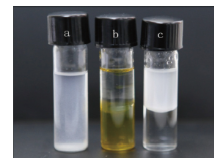
The dispersion performance of amphiphilic nanoparticle in water and organic solvent was studied (Fig.6). The modified silica material was found well-dispersed in hexane environment, indicating that amphiphilic silica particles were highly lipophilic. To facilitate the observation, a small amount of methyl orange was added to color the water. Silica nanoparticles of 1% mass fraction was added to the water and hexane mixed system (water and hexane volume ratio is 1:1). It showed that the modified silica nanoparticles were well dispersed in the hexane phase.
3.4.1 Effect of Weight Ratio of Unmodified Silica Sphere to OTS
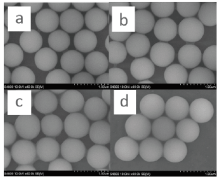
The images acquired using the SEM (Fig.2) showed that as the weight ratio of SiO2: OTS increased, adhesive ability between particles improved. In order to find the most effective amphiphilic silica particle for detecting latent fingerprint, the effectiveness of 4 different weight ratios (100׃1, 50׃1, 10׃1, 2׃1) was compared. All types of modified particles were used to detect fresh fingerprints, which were left on glass surfaces (Fig.7). Even though all of modified nano-silica particlesʼ powder appeared to be able to detect latent fingerprints, the best weight ratio of SiO2: OTS was 10:1.
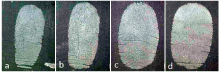 | Fig.7 Images of latent fingerprints developed by amphiphilic silica particle which was modified by the SiO2: OTS weight ratio of 100:1 (a), 50:1 (b), 10:1 (c) and 2:1 (d) |
3.4.2 Effect of Particle Diameter
The diameter of the amphiphilic silica particles depended on the hydrophilic silica material used. SiO2 nano-particles prepared by the Stober method usually had uniform spherical surface with a large number of bonded hydroxyl groups at different states (e.g., isolated hydroxyl, hydrogen bonded hydroxyl and twins hydroxyl). Many factors might influence the preparation of the monodisperse silica spherical particles, such as the type of catalyst, the concentration of silicon source, the type of solvent, hydrolysis temperature, and reaction time. In the case of using ammonia as a catalyst, its concentration was the main factor influencing the particle size, followed by the silicon source density.
Different sizes of amphiphilic silica particles (250, 550 and 700 nm) were synthesized. Results showed that both the size of the nanoparticles and the weight ratio of SiO2: OTS could affect the fingerprint detection. The best diameter for the amphiphilic silica particles (DASP) was 700 nm and best weight ratio of SiO2: OTS was 10:1 (Table 1).
| Table 1 Summary of the results obtained when different sets of latent fingerprints on glass slices developed with different weight ratio of SiO2: OTS and different diameter of modified silica particles |
3.4.3 Results of Fingerprints Development
The modified amphiphilic silica particles with a diameter of 700 nm and a weight ratio of SiO2: OTS at 10:1 were used to develop latent fingerprints on nonporous objects such as glass, ceramic and plastic. With a subtle size, the high lipophilic particle could absorb onto the fingerprintsʼ oil residual much well. Compared with the traditional powders as golden, silver and magnetic powder, the amphiphilic silica nanoparticle could generate much more recognizable fingerprint image with continuous ridges and visible pores (Fig.8).
A new type of modified amphiphilic silica nanoparticles was synthetized using OTS, and was successfully applied to detect fresh and aged latent fingerprints on slide glass. Such particles are very sensitive and selective to amino acids due to their good lipophilic and hydrophilic properties. Different particle sizes and SiO2:OTS weight ratios were investigated to optimize the formulation of the nano-particles. Silica sphere with a diameter of 700 nm and modified at a weight ratio of 10:1 showed the best performance when used to detect and develop latent fingerprints. These findings enable to propose a method for latent fingerprints detection on smooth nonporous objects. The wider range of applications of the new powder is currently under investigation, as well as the use of other types of silica nanoparticles for the detection of latent fingerprints on various other surfaces.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|