作者简介:鲍立垠(1986—),男,江西南昌人,助理研究员,博士,研究方向为枪弹检验。 E-mail: boilingbao@163.com
目的 枪弹射击时间估算是法庭科学物证研究的重要方面,能够为涉枪案件侦破和法庭诉讼提供不可替代的物证。近年来国外对此研究报道逐渐深入,而国产枪弹的射击时间研究尚属起步阶段。现代枪支的射击往往伴随着含氮发射药燃烧产生多种气相和固相射击残留物的过程,射击后可挥发的射击残留物随时间从枪管中不断逸散,对这种逸散过程的测量是估计发射时间的基础。随着化学分析手段的发展,近年来发展起来的固相微萃取技术,在用于分析化学领域的同时,因为其精准和低检出限,也引发了法庭科学枪弹领域研究者的关注。使用固相微萃取进行弹壳空腔内非接触采样,通过气相色谱进样,并使用热能分析仪、质谱等检测器对样品进行定量/半定量分析,是目前推断枪弹射击时间的主要方法;国产枪支和枪弹的发射时间问题,尚无通过这一方法进行的研究报道。方法 射击用枪选用54式7.62 mm手枪和国产12号唧筒式猎枪,实验枪弹选用国产51式7.62 mm手枪弹和嘉陵牌12号猎枪弹。射击实验后的射击弹壳在室温、通风条件下保存,保证射击弹壳内的挥发性有机射击残留物能正常挥发。在随后的分析测试中,以射击实验到取样测试的时间为射击时间。通过固相微萃取提取国产51式7.62 mm手枪弹和嘉陵牌12号猎枪弹射击弹壳空腔内的挥发性含氮有机残留物,使用气相色谱-热能分析仪进行分析,测量含氮有机物相对含量随时间的推移变化,并使用这一含量参数变化估测射击时间。结果 在12号猎枪弹射击弹壳从射击后1 d到9 d的气相色谱-热能分析谱图中,1~2 min之间均能观察到一个显著的双峰,且这个双峰随着射击时间的增加而不断减弱。通过对谱图的运算处理,得到含氮有机物峰的峰面积和峰高,分别得到与射击时间的关系,不论是峰面积还是峰高,都随射击时间的增长表现出降低的趋势。在11 d所测得的谱图中,也能观察到有两个峰值,但已变得微弱且不明显,峰面积与峰高接近于0。结果说明12号猎枪弹射击弹壳中不仅存在挥发性含氮有机物,而且其含量会随时间不断减少。对于51式手枪弹弹壳,在相同的取样分析方法获取的谱图中,相同时间范围内没有出现类似的显著谱峰,说明51式手枪弹的发射过程没有形成可供分析测量的含氮有机物。对于不同种类枪弹,挥发性含氮物质存在并得以检出,是使用气相色谱-热能分析仪进行发射时间估算的前提。不论是枪支还是枪弹的射击时间研究,尽管能采用相同或类似有机物残留量的气相色谱分析方法,但其变化趋势不同,因此必须针对不同品种、规格和品牌的枪支、枪弹分别得出有机物残留量变化曲线。结论 建立了通过固相微萃取手段,对射击弹壳中有机射击残留物取样,并使用气相色谱-热能分析仪进行含氮有机物总量测试的方法,首次实现对国产枪弹射击弹壳的含氮有机射击残留物随时间衰减变化的无损检测。得出嘉陵牌12号猎枪弹含氮物质峰的面积和峰高与射击时间的关系曲线,该曲线可用于估计该种枪弹的发射时间。从51式7.62 mm手枪弹中未检出含氮有机物残留,气相色谱-热能分析法不适用于51式7.62 mm手枪弹的射击时间估计。
Objective The time lapse of spent cartridge usually plays an important part in gunshot crime investigation. This study conducted quantificational analysis of volatile nitrate organics for estimation of the discharge time of Chinese-made cartridges.Methods Volatile nitrate organic products on the internal of fired cartridge cases, shotgun shells and gun barrels diffused into air slowly with time. After shooting, 7.62 mm model 51 pistol cartridges and 12/70 JIALING shotgun shells were sampled with solid phase micro-extraction (SPME) since discharge from day 1 and day 9, and analyzed by gas chromatography-thermal energy analyzer GC-TEA in order to detect the volatile nitrate organic of gunshot residues.Results One notable double peak detected from fired 12/70 JIALING shotgun shells was observed in GC-TEA chromatogram. The observed double peak became weaker along with the increasing time since discharge, and it became too weak to be identified on day 11. Not only the existence of volatile nitrate organic compounds but also the decrease of its amount was indicated in the analysis. However, the similar double peak of volatile nitrate organic compounds, observed in 12/70 JIALING shotgun shells, was not existent at all in 7.62 mm model 51 pistol cartridges. The content of volatile nitrate organics, generated during shooting, was not enough for GC-TEA test.Conclusions For the first time, the variety curves of the volatile nitrate organic compound content and the time since discharge of cartridges made in China was obtained. The test method of GC-TEA could be used in the discharge time estimation of the 12/70 JIALING shotgun shells, but not in that of the 7.62 mm model 51 pistol cartridges. For different types of cartridge, the concentration of volatile nitrate organic compound extracted from the shell has to be detectable with SPME-GC-TEA, prior to estimating the time since cartridge discharge.
Estimation of discharge time for firearms or cartridges is an important part of firearm-related crime investigation. Accompanied by shooting, gunpowder in cartridges burns and reacts vigorously, and volatile nitrate organic compounds generated in the internal gunshot cases and barrels diffuse into air over time. Qualitative test was used to infer whether the cartridge cases or guns were fired and the discharge time [1]. Sinha summarized the dating methods of firing time. The gunshot case mouth was covered by filter paper treated with absorbent reagent consisted of L-naphthylamine and sulfanilic acid. The chemical reactions between reagent and nitrate organics synthesized during firing process presented as pink color on filter paper, which was measured and compared with time length in order to estimate the discharge time [2]. Sampling by wiping and washing of the fired barrels or cases, would destroy the original sample instead of improving the gaseous recovering rate [3].
The solid phase micro-extraction (SPME), a nondestructive sampling technique, was deployed by Andrasko et al. to retrieve volatile organic firing residues inside the barrel and cartridge[3, 4, 5, 6, 7]. Gas chromatography-thermal energy analyzer (GC-TEA) or gas chromatography-flame ionization detector (GC-FID) was integrated to measure the lapsed time since a firearm or cartridge was fired. Nitrogenous compounds analysis with GC-TEA could achieve total nitrogen content, via high temperature splitting decomposition of nitrate organics coupled with chemiluminescence detection. One GC-TEA peak, decreased with time lapse, was considered to be closely related to the concentration of nitro- and nitroso- organic compounds. Naphthalene detection using GC-FID also presented the decay of organic gunshot residue over time, to verify the potential of the lapsed time assessment. Later, discharge time of rifles and pistols was further studied using different types of ammunition, while comparing GC-TEA to GC-FID. It was concluded that the analysis of total nitrate content was more reliable for discharge time estimation, due to the fact that naphthalene was quick to escape, at low content and over-relied on different types of cartridge, though under certain circumstances, naphthalene detection was more reliable and undisturbed than that of total nitrate content. Additionally, it was necessary for the separate measurement to draw variation curve of organic gunshot residue (GSR) on every type of firearm and cartridge.
Wilson JD et al. monitored naphthalene content decay in fired shotgun shells using gas chromatography-mass spectrometry (GC-MS) [8]. Airtight glass containers were used to preserve the shotgun shell, in order to prevent the escape of volatile organic of GSR. SEMP was carried out through cork of container, during which the non-contact operation could be achieved.
Weyermann et al[9]. analyzed GSR of 9 mm Luger pistol ammunition using GC-TEA and 32 organic compounds were detected, 6 kinds of compound could be used to estimate the emission time. Among them, benzonitrile, phenol, 2-ethyl-1-hexanol and naphthalene decayed rapidly as result of quick volatilization, short residence time, and only extremely low content remained 2 hours after firing, while 1, 2-dicyanobenzene and diphenylamine both containing a great quantity of nitrogen containing groups, could be determined 32 hours later. This study indicated that GC-TEA was practicable for discharge time estimation by analysis of total volatile nitrate organic contents.
Qualitative analysis of discharge time is dominant in Chinese forensic community, such as identification of marks on cartridge and observation of gunshot residue particles with scanning electron microscope [10, 11]. There is barely relevant study on the quantitative/semi-quantitative analysis of time since discharge of Chinese homemade firearm using GC/MS, although it has already drawn more and more attention [12]. In the present study, SPME-TEA is employed to analyze volatile nitrate organic compounds in common cartridges made in China under simulant open and natural volatilization environment. With the content decay pattern monitored, the discharge time are discussed.
Shooting experiment was carried out with pistol model 54, caliber 7.62 mm with 7.62 mm model 51 pistol cartridge, and pump shotgun, caliber 12/70 with 12/70 JIA-
LING cartridges, all made in China. After shooting, the fired cases were kept at room temperature and under aeration, ensuring the normal volatilization processes of volatile nitrate organic inside the shooting cases. In the following analysis, the time length between shooting and extraction was defined as discharge time of the special case.
Fused silica fibers with an 85 μ m polyacrylate coating (PA. PN:57304) purchased from Sigma-Aldrich (USA) was used in the experiments. Prior to the examinations, new fused silica fibers should be activated in the injection port of GC at 250℃ for 2 hours, and then a blank run was performed to make sure the cleanness and stability of fused silica fibers. After every injection, each silica fiber used would be kept in the injection port of GC at 180℃ for at least 7 min, which would free from interfering substances. During the extraction, the fused silica fiber was pushed inside the fired cartridge case near the geometrical center of case cavity for 20 min, without contacting with the inner surface. SPME was performed inside 7.62 mm model 51 pistol cartridge cases and 12/70 JIALING shotgun shells at day 1, day 3, day 7, day 9 and day 11 for the further analysis.
GC-TEA system was comprised of Agilent 7890B GC and Ellutia 800 Series TEA with moderate polar glass capillary columns of DB-6MS were chosen. The temperature rose to 200℃ at the heating rate of 50℃ per minute after injection at 180℃, and lastly reached at 270℃. The data in 10 min after injection were used in further analysis. Thermal energy analyzer was set at the work pattern of total nitrate content measurement, and the splitting decomposition temperature was 850℃, by which all nitro-, nitroso- and nitric acid ester groups would be totally decomposed and the nitrate content measurement would be achieved.
SPME sampling was undertaken to collect GSR from 7.62 mm model 51 pistol cartridge cases and 12/70 JIA-
LING shotgun shells 1 day after discharge, and the extracts were analyzed by GC-TEA. The result was shown in Fig.1. One marked double peak was observed on the chromatogram of 12/70 JIALING shotgun shells in the time segment from 1 to 2 min of GC-TEA analysis. Under nitrate pattern, the thermal energy analyzer was unable to determine the kind of organic compounds. Instead, the nitro-, nitroso- and nitric acid ester groups in volatile nitrate organics all decomposed at high temperature and generated NO and NO2 which were available for the following photoelectric detection. As a consequence, the peak of 12/70 JIALING shotgun shell in Fig.1 should come from the nitrate groups of the volatile organic compounds extracted by SPME from cartridge case. The relative strength of the nitrate peak could be used to represent the relative contents of the nitrate organics.
In the same diagram, no prominent peak was observed during the test time frame for the model 51 pistol cases. It could be inferred that the amount of volatile nitrate organics was not synthesized enough for the detection with GC-TEA when the cartridge was fired. For model 51 pistol cartridges, it will not be possible to determine the time interval of GSR deposition. Therefore, the subsequent test of discharge time estimation was only performed on 12/70 JIAING shotgun shells with marked peak monitored, with the purpose to clarify the relationship between the discharge time and the GC-TEA peak value observed above.
 | Fig.1 GC-TEA chromatogram of GSR collected from 12/70 JIA-LING shotgun shell (up) and from 7.62 mm model 51 pistol cartridge (down) 1 day after shooting |
SPME sampling was carried out from the same 12/70 JIALING shotgun shell 1, 3, 7, 9 and 11 days after shooting respectively, and followed with the GC-TEA analysis, of which the results were shown in Fig.2. All samples over the time frame of experiment had a double peak observed in the GC-TEA test time segment of 1 to 2 min. The shape of the double peak from earlier days was higher and sharper, while the peak shape from later ones became lower and broader. Under the same coordinate system, it was notable that the double peak representing nitrate organics revealed the decreasing pattern when the discharge time increased. On day 11, its chromatographic double peak became too weaker to be noticeable during the same test time range.
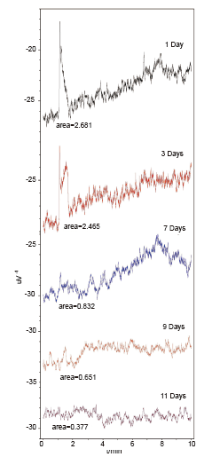 | Fig.2 GC-TEA chromatograms of GSR collected from 12/70 JIA-LING shotgun shell 1, 3, 7, 9 and 11 days after shooting |
It could be inferred from the repeated appearance of the nitrate peak in GC-TEA chromatogram that the volatilization and diffusion processes of nitrate organics inside the 12/70 JIALING shotgun shells existed over 11 days. As time went on, volatile nitrate organics adsorbed on the metallic interface of fired cases escaped, and less and less nitrate organics measurable with SPME-GC-TEA remained. Thus the above all well explains the decreasing trend of nitrate peak with the increase of discharge time.
The key point for the discharge time estimation is to find out a particular parameter, which will change significantly with time, and the estimated value of discharge time could be obtained by the measurement of this parameter. If the decreasing behavior of nitrate organics peak, demonstrates a certain regular pattern, as what observed in GC-TEA test of 12/70 JIALING shotgun shell, it would be possible to estimate the time since discharge of ammunition. With the GC-TEA data processed, the area and height of the nitrate peak of 12/70 JIALING shotgun shells were obtained, and the curve fitting lines were shown in Fig.3 and Fig.4.
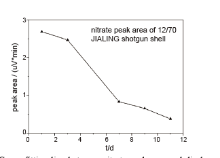 | Fig.3 Curve-fitting line between nitrate peak area and discharge time of 12/70 JIALING shotgun shell |
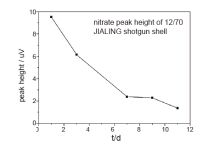 | Fig.4 Curve-fitting line between nitrate peak height and discharge time of 12/70 JIALING shotgun shells |
No matter peak area or height, the variation tendency was the same, with the discharge time value decreased with the time. On day 11 after discharge of cartridge, the organic compound peak became too weak to be visible, and the peak area and height fell down to zero. The variety curve of peak area and peak height along with time could represent the variety of nitrate organic residue content in 12/70 JIALING shotgun shell after shooting, and the time estimation could be possible over 11 days since the similar type of shotgun shell was fired. As the peak area and peak height only stand for the relative content of nitrate organics, other than the absolute value, the direct comparison of nitrate organics measurement is not accurate, In addition, the decrease trend of nitrate compoundsis also closely related to the type of ammunition. Therefore, the standard curve describing nitrate organic content variety under similar firing condition has to be drawn, every time prior to estimation of cartridges discharge time.
In the present study, non-destructive SPME sampling coupled with GC-TEA was first employed in Chinese-made cartridges, to determine the decrease pattern of organic nitrate residue along with time. Nitrate organic shooting residue was detected from fired 12/70 JIALING shotgun shells, which generated one notable GC-TEA double peak in the time segment of 1 to 2 min. The peak decayed gradually as the discharge time extended, until the nitrate peak disappeared at all on day 11 after shooting. The curve-fitting line between discharge time of 12/70 JIALING shotgun shells and nitrate peak area or height was obtained, by which the shooting time of this type of cartridge could be estimated. There was no result from fired model 51 pistol cartridges, which indicates that the GC-TEA approach is not suitable for the discharge time estimation of the model 51 pistol cartridges. In general, the measureable concentration of volatile nitrate organic compounds retrieved from ammunition should be the prerequisite of discharge time estimation for different types of cartridges.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] | [本文引用:1] |
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|


