作者简介:刘晓(1989—),女,四川自贡人,硕士研究生,研究方向为传染性疾病的流行病及微生物、生物毒素、违禁药品的快速检测。E-mail:liuxiao042889@126.com
目的 利用上转换发光免疫层析技术(UPT-LF)建立一种尿液中吗啡(MOP)及甲基苯丙胺(MET)快速定量检测方法并对其进行系统评价。方法 以上转换发光纳米颗粒(UCP-NPs)作为生物示踪物,竞争模式免疫层析作为检测平台,建立可对尿液中MOP及MET进行定量检测的UPT-LF,即MOP-UPT-LF、MET-UPT-LF。以MOP-UPT-LF为代表,评价UPT-LF对痕量毒品检测极限,通过系列浓度标准品测定,评价定量检测能力。根据常规检测阈值,调整MOP-UPT-LF及MET-UPT-LF检测敏感性及线性范围,并评价其定量检测能力。以LC-MS、GC-MS分别作为MOP、MET检测的金标准,以胶体金免疫层析作为对照,对执法现场收集尿样进行检测,确定UPT-LF定性检测性能。对系列浓度MOP模拟阳性样本同时进行LC-MS及MOP-UPT-LF定量检测,对系列浓度MET模拟阳性样本同时进行GC-MS及MET-UPT-LF定量检测,评价UPT-LF定量检测性能。结果 在痕量检测条件下,MOP-UPT-LF敏感性可达1ng/mL,线性范围为1~5000ng/mL(r=-0.98172,P<0.0005)。在常规检测条件下,MOP-UPT-LF敏感性为50ng/mL,线性范围调整为50~3000ng/mL(r=-0.98464,P<0.0005);MET-UPT-LF敏感性为100ng/mL,线性范围为100~5000ng/mL(r=-0.99964,P<0.0005)。就定性检测而言,MOP-UPT-LF及MET-UPT-LF均较优,灵敏度及特异度均为100%,与胶体金结果一致。就定量检测而言MOP-UPT-LF及MET-UPT-LF与定量确证方法LC-MS及GC-MS无显著差异。结论 本研究建立MOP-UPT-LF、MET-UPT-LF方法,在满足快筛试剂快速简便的基础上,进一步实现了现场快速定量检测,为尿液中毒品的现场快速定量检测提供了技术保障。
Objective To develop and evaluate an up-converting phosphor technology based on lateral flow assay (UPT-LF) for qualitative and quantitative detection of morphine (MOP) and methamphetamine (MET) in urine.Methods With up-converting phosphor nano-particles (UCP-NPs) as the biological tracer, two competitive mode-based LF strips, MOP-UPT-LF and MET-UPT-LF were developed for quantitative detection of MOP and MET in urine. The comprehensive performances of MOP-UPT-LF and MET-UPT-LF were evaluated systematically. In order to explore the detection limit of UPT-LF for trace analysis, MOP-UPT-LF strips were used to test standard samples with series of concentrations, and then the detection limit and ability of quantitative detection were determined. According to the standard of detection threshold for MOP and MET, the quantitative detection performances (including detection sensitivity and linear range) of MOP-UPT-LF and MET-UPT-LF were optimized and re-evaluated. For the evaluation of qualitative detection ability, the results of MOP-UPT-LF and MET-UPT-LF for on-site urine samples were compared with those of colloidal gold based LF (CG-LF), and LC-MS and GC-MS were used as the gold standard for the detection of MOP and MET, respectively. For the evaluation of quantitative detection ability, the results of MOP-UPT-LF and MET-UPT-LF for simulated positive urine samples were compared with those of LC-MS and GC-MS, respectively.Results For trace analysis, the detection limit of MOP-UPT-LF could reach 1ng/mL with a linear range from 1ng/mL to 5000ng/mL (r = -0.98172,P < 0.0005). For routine detection with the threshold of standard, the detection limit of MOP-UPT-LF was 50ng/mL with a linear range from 50ng/mL to 3000ng/mL (r = -0.98464,P < 0.0005). The detection limit of MET-UPT-LF was 100ng/mL with a linear range from 100ng/mL to 5000ng/mL (r = -0.99964, P < 0.0005). According to the detection of urine samples, the performance of qualitative and quantitative detection of MOP-UPT-LF and MET-UPT-LF could meet the need of the on-site rapid detection of MOP and MET in urine. The qualitative detection results of MOP-UPT-LF and MET-UPT-LF for on-site urine samples were consistent with those of colloidal gold and no false-positive and false-negative results observed. The ROC area of MOP-UPT-LF and MET-UPT-LF reached 1.000±0.000 (95%CI). For quantitative detection of simulated positive urine samples, the recovery rate was 77%~133% with the mean of 109% and CV of 21% for MOP-UPT-LF, for MET-UPT-LF the recovery rate was 80%~131% along with the mean of 112% and CV of 17%. After statistical analysis, there was no significant difference (P>0.05) between MOP-UPT-LF/MET-UPT-LF and LC-MS/GC-MS for quantitative detection of MOP and MET in urine.Conclusions With the novel optical nano-particle (UCP-NPs), the traditional LF assay was integrated with biosensor based on automated analysis and two kinds of UPT-LF strip were developed to meet the need of on-site qualitative and quantitative detection of MOP and MET. With GC-LF as the reference for qualitative detection and LC-MS/GC-MS for quantitative detection, the comprehensive performances (including detection limit, qualitative accuracy, and quantitative ability) were evaluated systematically. The good qualitative and quantitative detection performance of MOP-UPT-LF and MET-UPT-LF offers a new choice for on-site drug screening.
《禁毒法》第二条规定:“ 本法所称毒品, 是指鸦片、海洛因、甲基苯丙胺(冰毒)、吗啡、大麻、可卡因以及国家规定管制的其他能够使人形成瘾癖的麻醉药品和精神药品” 。在国家食品药品监督管理总局2012年发布的《国家药物滥用监测年度报告》中指出:海洛因与“ 冰毒” 是主要滥用物质, “ 冰毒” 滥用者有增长趋势。吗啡(Morphine, MOP)是鸦片重要成分, 也是海洛因在尿液中的代谢物质。甲基苯丙胺(Methamphetamine, MET)因其外观似冰故俗称为“ 冰毒” , 是一种具有强烈兴奋中枢神经作用的苯丙胺类物质, MET摄入体内后, 大部分以原形从尿中排出[1]。
毒品检测对吸毒行为的认定、戒毒治疗过程的监控具有重要作用, 是检察起诉、法院量刑的重要依据, 对打击毒品犯罪、开展禁毒戒毒工作具有重要意义[2, 3]。目前, 毒品的检测主要包括确证与初筛两种类型。确证方法主要包括色谱法、光谱法、质谱法等[4, 5, 6, 7, 8, 9, 10, 11, 12], 可实现定量检测, 且结果可靠, 但需严格的质控规程、标准的样品处理及精密的仪器分析, 其方法复杂对于操作人员与操作环境依赖性过强。为满足现场大量样品的快速筛查, 近年来各种初筛技术不断涌现, 其中最为成熟的技术是胶体金免疫层析[13]、酶联免疫[14]等, 可实现快速定性或半定量检测, 但现场初筛操作下的全定量检测尚未实现。针对这一问题, 本研究以免疫层析技术为基础, 将新型上转换发光纳米材料(Up-converting Phosphor Nano-Particles, UCP-NPs)[15, 16]作为生物示踪物与其有机结合, 采用竞争免疫模式, 研制可对尿液MOP及MET进行精确定量检测的两种上转换发光免疫层析(Up-converting Phosphor Technology based Lateral Flow, UPT-LF)试纸。本研究所建立的方法同时兼顾了现场条件下“ 操作的简便、快捷” 以及“ 结果的精确定量” , 从而为警方对涉嫌吸毒人员进行现场快速筛查提供了可靠便捷的技术手段。
UCP-NPs(NaYF4: Yb3+, Er3+, 粒径50nm, 激发光980nm, 发射光541.5nm)由上海科研光电科技有限公司制备。硝酸纤维素膜(SHF1350225)、玻璃纤维(GFCP20300)、吸水纸及粘性底衬购自美国Millipore公司; 试纸条塑料外壳由军事医学科学院微生物流行病研究所分析微生物实验室设计, 深圳金灿华公司制造。小牛血清白蛋白(BSA)、蔗糖、聚乙烯醇(PVA)及十二烷基磺酸钠(SDS)等生化试剂购自美国Sigma-Aldrich; MOP、MET标准品购自公安部物证鉴定中心; MOP、MET单克隆抗体及其与BSA交联抗原购自杭州隆基生物技术有限公司。胶体金试剂盒购自艾康生物技术(杭州)有限公司。尿液样品为现场执法时采集, 吗啡阳性尿液样品经LC-MS确证, 冰毒阳性尿液样本经GC-MS确证。
UPT生物传感器(上转换发光免疫分析仪, UPT-3A型)由军事医学科学院微生物流行病研究所与中国科学院上海光学精密机械研究所联合研制。其它主要设备包括:5417R高速离心机(Eppendorf), 喷膜机(Imagene Echnology), DHG-9245A烘干机(上海一恒科学仪器), ZQ4000数控高速斩切机(上海金标生物科技有限公司)。
对MOP进行痕量检测(1ng/mL)的MOP定量检测UPT-LF试纸(MOP-UPT-LF)的制备方法如下。样品垫制备:吸水纸于样品垫处理液(0.3mol/L pH7.2磷酸盐缓冲液含0.5%SDS、1%BSA、0.5%PVA)中浸泡10min, 取出后37℃烘干2h, 备用; 结合垫制备:将UCP-NPs与抗MOP单克隆抗体、羊IgG分别共价偶联[17], 用结合物稀释液(0.03mol/L pH7.2磷酸盐缓冲液含0.01%SDS、0.5%PEG20000、1%BSA)将UCP-抗MOP单克隆抗体结合物与UCP-羊IgG结合物等比混合, 以终浓度0.2mg/mL泡玻璃纤维5min, 45℃下烘干50min, 备用; 分析膜制备:将终浓度0.5 mg/mL MOP-BSA与1mg/mL兔抗羊IgG, 按2µ L/cm喷点于分析膜上作为检测带(T)与质控带(C), 45℃下15min烘干, 备用。将样品垫、结合垫、分析膜、吸水垫依次贴于底衬上, 4mm/条斩切后放入塑料外壳, 备用。
对MOP、MET进行常规阈值浓度(MOP:300ng/mL; MET:1000 ng/mL)检测的MOP定量检测UPT-LF试纸(MOP-UPT-LF)与MET定量检测UPT-LF试纸(MET-UPT-LF)制备方法如下。样品垫制备:MET-UPT-LF样品垫处理液为0.3mol/L pH7.2磷酸盐缓冲液含0.5%SDS、1%BSA、0.5%PEG8000、0.1%NaCl, 其余同上; 结合垫制备:UCP-抗MOP/MET单克隆抗体结合物与UCP-羊IgG结合物等比混合, 以终浓度0.5mg/mL浸泡玻璃纤维, 其余同上; 分析膜制备:将终浓度1.5mg/mL MOP-BSA/MET-BSA与2mg/mL兔抗羊IgG喷点于分析膜上, 其余同上。
100µ L尿液样品直接添加于试纸条样品垫部分, 静置层析15min。用UPT生物传感器对分析膜进行扫描分析, 获得检测带对应的T值与质控带对应的C值, 以T/C作为检测结果。检测的过程中待检MOP/MET与T带上的MOP-BSA/MET-BSA竞争结合UCP-抗MOP/MET抗体复合物, 从而形成样品中MOP或MET浓度与T/C值呈反比的量效关系, 若待检样品T/C值小于Cutoff值(空白均值-3× 标准差), 则样品判定为阳性, 反之为阴性。
以尿液中MOP检测为例, 不考虑实际应用中的检测阈值, 确定在违禁品检测中UPT-LF方法可达到的最高检测灵敏度。以阴性尿液分别配制系列浓度MOP标准品, 浓度为0ng/mL、1ng/mL、10ng/mL、100ng/mL、1000ng/mL、3000ng/mL、5000ng/mL, 每个浓度样品重复测试三次, 以阴性尿液(0ng/mL)作为空白对照, 以T/C值的均值-3SD(
以《国际药物滥用监测标准》为参考, 结合我国国情及常规执法检测阈值(MOP:300ng/mL; MET:1000ng/mL), 对MOP-UPT-LF及MET-UPT-LF检测敏感性、线性范围进行调整后, 以阴性尿液配制MOP、MET模拟样品, MOP系列浓度为0ng/mL、50ng/mL、150ng/mL、300ng/mL、450ng/mL、1000ng/mL、3000ng/mL, MET系列浓度为0ng/mL、100ng/mL、1000ng/mL、3000ng/mL、5000ng/mL, 每个浓度样品重复测试3次, 以阴性尿液(0ng/mL)作为空白对照, 以T/C值的均值-3SD(
现场采集涉嫌吸毒人员尿液, 包括97份吗啡现场筛查尿样及30份冰毒现场筛查尿样, 吗啡筛查尿液样品经LC-MS确证, 其中41份为吗啡阳性, 56份为吗啡阴性; 冰毒筛查尿液样本经GC-MS确证, 其中22份为冰毒阳性, 8份为冰毒阴性。将上述尿液, 100µ L/条直接仪器上样, 分别进行MOP-UPT-LF及MET-UPT-LF检测, 同时按试剂盒标准操作进行胶体金试纸条检测, 胶体金检测MOP阳性阈值为300ng/mL、MET阳性阈值为1000ng/mL。分别以LC-MS及GC-MS检测结果作为金标准, 比较UPT-LF与胶体金两种方法定性检测一致性, 评价MOP-UPT-LF及MET-UPT-LF对现场尿样的定性检测能力。
以阴性尿样分别加入MOP及MET标准品, 配制系列浓度模拟阳性样品, MOP模拟样品系列浓度为50ng/mL, 150ng/mL, 300ng/mL, 450ng/mL, 1000ng/mL, 3000ng/mL; MET模拟样品系列浓度为125ng/mL, 500ng/mL, 1000ng/mL, 1500ng/mL, 3000ng/mL。对MOP模拟阳性样本同时进行LC-MS及UPT-LF定量检测, MET模拟阳性样本同时进行GC-MS及UPT-LF定量检测, 分别比较两种检测方法定量结果一致性, 评价MOP-UPT-LF/MET-UPT-LF对尿液中MOP/MET定量检测性能。
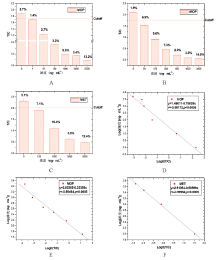
以浓度(ng/mL)作为横坐标(x), T/C值为纵坐标(y), 获得浓度响应图(见图1), 图中百分比代表各浓度重复测量变异系数; 结果显示, 以阴性样品的
随着MOP、MET浓度增加, T/C值降低, 以Logit(T/C)作为横坐标(x), Log(浓度[ng/mL])作为纵坐标(y), 获得标准定量曲线(见图1), 经统计拟合分析可知在痕量检测条件下, MOP-UPT-LF对MOP进行检测在1~5000ng/mL之间具有线性关系(r = -0.98172, P < 0.0005)(见图1D); 按常规检测需求调整后, MOP-UPT-LF对MOP进行检测在50~3000ng/mL之间具有线性关系(r = -0.98464, P < 0.0005)(见图1E), MET-UPT-LF对MET进行检测在100~5000ng/mL之间具有线性关系(r = -0.99964, P < 0.0005)(见图1F)。
按试剂盒标准操作流程对现场尿液样品进行胶体金检测。在UPT-LF检测中, 100µ L尿样直接上样检测, 将上述常规检测阈值敏感性评价中
| 表1 现场尿样MOP-UPT-LF及MET-UPT-LF定性检测评价 Table 1 Qualitative detection of MOP-UPT-LF and MET-UPT-LF in on-site urine samples |
如表1所示, 对于尿液中MOP及MET的定性检测, MOP-UPT-LF及MET-UPT-LF与胶体金结果一致, 均无假阳性及假阴性结果出现, 检测效能较优。如图2所示, MOP-UPT-LF(见图2A)检测尿液中MOP, ROC曲线下面积为1.000± 0.000(95%CI), MET-UPT-LF(见图2B)检测尿液中MET, ROC曲线下面积为1.000± 0.000(95%CI), 诊断准确性均较优。
采用LC-MS/GC-MS与MOP-UPT-LF/MET-UPT-LF对模拟阳性样品进行定量检测, 分别根据其定量曲线计算样品中MOP及MET回归浓度, 结果见表2。就尿液中MOP检测而言, MOP-UPT-LF检测回收率在77%~133%之间, 均值与CV分别为109%、21%, LC-MS检测回收率在80%~131%之间, 均值与CV分别为112%、17%; 就尿液中MET检测而言, MET-UPT-LF检测回收率在81%~119%之间, 均值与CV分别为105%、12%, GC-MS检测回收率在56%~101%之间, 均值与CV分别为85%、19%。将MOP-UPT-LF与LC-MS的回收率进行配对t检验, t = 0.591, P > 0.05; 将MET-UPT-LF与GC-MS的回收率进行配对t检验, t = 2.271, P > 0.05; 即就定量检测结果而言MOP-UPT-LF及MET-UPT-LF与传统确证方法LC-MS及GC-MS无显著性差异, 且就平均回收率而言, MOP-UPT-LF(109%)及MET-UPT-LF(105%)略优于定量确证方法LC-MS(112%)及GC-MS(84%), 其检测性能可满足现场快速定量检测要求。
| 表2 尿液中MOP-UPT-LF/MET-UPT-LF检测回收率 Table 2 Recovery rate of MOP-UPT-LF and MET-UPT-LF in urine sample |
近年来伴随体外诊断技术的迅猛发展, 缩短现场初筛技术与实验室确证技术之间的性能差异, 提高现场执法准确性是大势所趋。胶体金免疫层析技术由于操作简便、价格低廉、无需辅助设备而广泛应用于现场筛查, 但其存在肉眼判读主观差异及检测结果无法有效保存的问题。本研究在承袭层析技术简便快捷的基础上, 采用具有独特上转换发光现象的UCP-NPs作为光学生物示踪物, 使免疫层析与生物智能传感有机融合, 实现了现场初筛条件下的自动仪器判读与精确定量, 使现场执法结果作为证据永久保存得以实现。经系统评价在痕量MOP检测中, MOP-UPT-LF敏感性可达1ng/mL, 在1~5000ng/mL之间具有线性关系(r = -0.98172, P < 0.0005); 在常规检测中, MOP-UPT-LF敏感性为50ng/mL, 在50~3000ng/mL之间可精确定量(r = -0.98464, P < 0.0005), MET-UPT-LF敏感性为100ng/mL, 在10~5000ng/mL之间可精确定量(r = -0.99964, P < 0.0005)。在定性检测评价中, MOP-UPT-LF及MET-UPT-LF灵敏度均为100%, 特异度均为100%, ROC曲线下面积均为1.000± 0.000(95%CI), 均具有较优检测效能。其定性检测能力与目前成熟快筛试剂胶体金一致, 且MOP-UPT-LF及MET-UPT-LF具有其他快筛试剂所不具备的定量检测能力, 与确证方法LC-MS及GC-MS定量能力比较无显著差异。MOP-UPT-LF与MET-UPT-LF操作简便, 样品无需前处理即可直接上样, 16min内可出检测结果, 具备快速、敏感性高、特异性好、可精确定量等诸多优点, 为打击毒品犯罪, 开展禁毒、戒毒工作提供了先进的毒品检测技术。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|